Answers
Answer:
the answer is 74.1 g/mol
Explanation:
Answer:
The molar mass of dimethylnitrosamine is 74.1 g/mol b
Related Questions
Which ocean zone lies inside V-shaped ocean trenches? O A. Bathypelagic O B. Abyssopelagic Ο Ο Ο Ο O C. Mesopelagic O D. Hadalpelagic
Answers
Answer:
O D. Hadalpelagic is the answer.
A solution of aluminum chloride has a pH of (4.5x10^0). What is the [H3O*(aq)], in mol/L?
Note: Your answer is assumed to be reduced to the highest power possible.
Answers
The concentration of H3O+ ions in the solution of aluminum chloride is \(3.16×10^-5\) mol/L.
Aluminum chloride is an acidic salt that contains a cation, Al3+, and an anion, Cl-. When aluminum chloride is dissolved in water, it dissociates into its constituent ions, and the Al3+ cations hydrolyze to produce H+ ions.
This reaction leads to the formation of an acidic solution. The pH of a solution of aluminum chloride is \(4.5×10^0\). We need to determine the concentration of H3O+ ions in this solution.
The concentration of H3O+ ions in a solution is given by the equation: pH = -log[H3O+] where pH is the negative logarithm of the concentration of H3O+ ions in the solution. The negative sign indicates that the pH is inversely proportional to the concentration of H3O+ ions. To determine the concentration of H3O+ ions, we need to rearrange the equation:
[H3O+] = \(10^-pH\) Substituting the value of pH =\(4.5×10^0\), we get: [H3O+] = \(10^-4.5\)
The value of \(10^-4.5\) can be calculated using scientific notation: \(10^-4.5\)= \(3.16×10^-5\) mol/L
for more such questions on concentration
https://brainly.com/question/28564792
#SPJ8
How many moles of NaCl can be produced from 2.5 moles of BaCl2.
Answers
2.5 moles of NaCl can be produced from 2.5 moles of BaCl\(_2\). Mole, often known as mol, is a commonly used unit of measurement.
What is mole?Mole, often known as mol, is a commonly used unit of measurement in chemistry for vast amounts of tiny objects such molecules, atoms, or other specific particles.
In addition, starting on May 20, 2019, the General Conference upon Weights and Measures declared the mole as the quantity of the International System of Units.
Na\(_2\)SO\(_4\) + BaCl\(_2\) → BaSO\(_4\) + NaCl
moles of BaCl\(_2\) =2.5 moles
the mole ratio between BaCl\(_2\) and NaCl is 1:1
mole of NaCl =2.5 moles
Therefore, 2.5 moles of NaCl can be produced from 2.5 moles of BaCl\(_2\).
To know more about mole, here:
https://brainly.com/question/15209553
#SPJ1
In the reaction 2 H, Ł02 → 2 H, O, what is the mole ratio of hydrogen to water?
A 2:2
B 2:1
C 1:2
D 4:4
Answers
A
because both have 2's in front of them
What solid figure has two bases, but no lateral faces?
cylinder
rectangular prism
triangular prism
cone
Answers
Answer:
it is cylinder as it has two bases but no lateral faces.
6. Balance the reaction
PCIS + H₂O → H3PO4 + HCI
Answers
The balanced chemical equation for the reaction is:
PCl5 + H2O → H3PO4 + HCl
To balance this equation, we need to make sure that the number of atoms of each element is equal on both sides of the equation.
To do this, we can start by balancing the phosphorus (P) and chlorine (Cl) atoms. There is one P atom and five Cl atoms on the left side of the equation, and one P atom and one Cl atom on the right side of the equation. We can balance these by adding a coefficient of 5 in front of HCl:
PCl5 + H2O → H3PO4 + 5HCl
Now we have five Cl atoms on both sides of the equation. Next, we can balance the hydrogen (H) atoms. There are two H atoms on the left side of the equation and five H atoms on the right side of the equation. We can balance these by adding a coefficient of 2 in front of H2O:
PCl5 + 2H2O → H3PO4 + 5HCl
Now we have two H atoms on both sides of the equation. Finally, we can balance the oxygen (O) atoms. There are two O atoms on the left side of the equation and four O atoms on the right side of the equation. We can balance these by adding a coefficient of 2 in front of H3PO4:
PCl5 + 2H2O → 2H3PO4 + 5HCl
Therefore, to balance this reaction, we need to use this balanced chemical equation:
PCl5 + 2H2O → 2H3PO4 + 5HCl
To know more about coefficient, visit :
https://brainly.com/question/28975079
#SPJ1
What Is The Molar Concentration Of Na+ Ions In 0.0200 M Solutions Of The Following Sodium Salts In Water?
Answers
The molar concentration of Na⁺ ions In 0.0200 M solutions of the given sodium salts in water is:
For NaBr, the molar concentration of Na⁺ ions = 0.020 M
For Na₂SO₄; the molar concentration of Na⁺ ions = 0.040 M
For Na₃PO₄; the molar concentration of Na⁺ ions = 0.060 M
What is the molar concentration of a substance?The molar concentration of a substance is the amount in moles of that substance that is present in a given volume of a solution of that substance.
Mathematically;
molar concentration = number of moles of substance / volume of the substanceThe molar concentration of Na⁺ ions In 0.0200 M solutions of the given sodium salts in water is calculated as follows:
Molar concentration = moles of sodium ions in 1 mole of the salt * molar concentration of the salt.
For NaBr;
moles of Na⁺ ions in 1 mole of the salt = 1 mole
molar concentration of Na⁺ ions = 1 * 0.020 M
molar concentration of Na⁺ ions = 0.020 M
For Na₂SO₄;
moles of Na⁺ ions in 1 mole of the salt = 2 moles
molar concentration of Na⁺ ions = 2 * 0.020 M
the molar concentration of Na⁺ ions = 0.040 M
For Na₃PO₄;
moles of Na⁺ ions in 1 mole of the salt = 3 moles
molar concentration of Na⁺ ions = 3 * 0.020 M
the molar concentration of Na⁺ ions = 0.060 M
Learn more about molar concentration at: https://brainly.com/question/26255204
#SPJ1
Complete question:
What Is The Molar Concentration Of Na+ Ions In 0.0200 M Solutions Of The Following Sodium Salts In Water?
NaBr
Na₂SO₄
Na₃PO₄
This is how manganese appears in the periodic table.
25
Mn
Manganese
54.94
What is the arrow is pointing to?
period symbol of manganese
isotope symbol of manganese
group symbol of manganese
Chemical symbol of manganese
Answers
The arrow is pointing to the symbol of the element. Option D
How do elements appear?We know that we arrange the elements that we have in the periodic table of the elements and that the properties of the elements could be revealed by the intricacy of the arrangement of the elements.
The entry of the elements in the periodic table does have three components and these are;
The symbol of the element
The mass number
The atomic number.
The arrow is showing what the chemical symbol of the manganese is.
Learn more about periodic table:https://brainly.com/question/11155928
#SPJ1

QUESTION
A sample of chromium chloride weighing 0.5000g is found to contain 0.3358g of chlorine. What is the empirical formula of the compound?
The molar mass of chromium is 51.9961 g/mol and the molar mass of chorine is 35.45 g/mol.
Answer choices:
-CrCI3
-CrCl
-Cr2Cl
-Cr2Cl3
Answers
The empirical formula is CrCl3.
What exactly are empirical formulas?
An empirical formula is a compound's chemical formula that only specifies the ratios of the elements it contains and not the precise number or arrangement of atoms. This would be the compound's element with the lowest whole number ratio.
Ionic compounds occur as crystal lattices containing cations and anions, as opposed to molecules. The empirical formula that identifies the proportion of cations present is already included in the chemical formula used to express an ionic compound like chromium (III) chloride. Each chromium (III) ion contains three chloride ions.
To learn more about empirical formula use link below:
https://brainly.com/question/1603500
#SPJ1
What is the molarity of a 0.5 L solution containing 20.0 grams of sucrose (molar mass of sucrose 342.3 g/mol)?
Answers
Answer:
Molarity of sucrose = 0.116 M (Approx.)
Explanation:
Given:
Volume of solution = 0.5 liter
Mass of sucrose in gram = 20 gram
Molar mass of sucrose 342.3 g/mol
Find:
Molarity of sucrose
Computation:
Mole of sucrose = Mass of sucrose in gram / Molar mass of sucrose
Mole of sucrose = 20 / 342.3
Mole of sucrose = 0.058 (Approx.)
Molarity of sucrose = Mole of sucrose / Volume of solution
Molarity of sucrose = 0.058 / 0.5
Molarity of sucrose = 0.116 M (Approx.)
Which of the following is a product of the reaction of a solution of potassium chloride mixed with a solution of lead (II) nitrate?

Answers
In this question, we have a reaction between two ionic compounds, Potassium chloride, KCl, and Lead (II) nitrate, Pb(NO3)2, and the complete and balanced reaction between these two compounds is the following:
Pb(NO3)2 (aq) + 2 KCl (aq) ---> PbCl2 (s) + 2 KNO3 (aq)
According to this balanced reaction, we can see that the only possible option for this question will be the solid PbCl2
Therefore the answer will be the 2nd option
write the structural formula for 6-Ethyl-4, 7-dimethyl-non-1-ene.
Answers
The structural formula for 6-Ethyl-4,7-dimethyl-non-1-ene can be represented as follows:
\(CH_{3} CH_{3} CH_{3}\)
| | |
\(CH_{2} CH_{2} CH_{2} CH_{2} CH_{2} CH_{2} CH_{2} CH_{2} CH_{2} CH_{3}\)
| | | | | | |
\(CH CH CH CH CH CH CH\)
|
\(CH_{2}\)
In this structural formula, the main chain contains nine carbon atoms (non-1-ene) with a double bond (ene) located at the first carbon atom. Starting from the first carbon atom, we have:
At the sixth carbon atom, there is an ethyl group (CH3CH2-), which means an ethyl group is attached to it.
At the fourth and seventh carbon atoms, there are methyl groups (CH3-), which means a methyl group is attached to each of them.
The remaining carbon atoms in the main chain have a single hydrogen atom (H) attached to them.
This structural formula represents the arrangement of atoms and bonds in the molecule and provides information about the connectivity of the atoms in the compound. It helps visualize the spatial arrangement of the atoms and functional groups, enabling a better understanding of the compound's chemical properties and reactions.
Know more about structural formula here:
https://brainly.com/question/31115811
#SPJ8
What is the job of a scientist why do scientits need government funding
Answers
2. Scientist need government funding in order to maintain prestige.
Answer:
1. A scientist conducts and gathers research to gain knowledge in a particular area.
2. Many of the worlds problems include resources, energy, health, environment, climate, transportation, communication, etc. and will require solutions from science and engineering.
9. Calculate the pOH and pH of a 0.35 M solution of the weak acid HCN.
Ka HCN = 4.8 x 10-10
Answers
Answer:
=0.35hcn20 4.7 x hcn(poh) =hcnty sa pointd
Does anyone know Chemistry
Answers
Answer:
so so
Explanation:
this your question?? <_>
22.5 mL of an HNO3 solution were
titrated with 31.27 mL of a
0.167 M Ca(OH)2 solution to reach the
equivalence point. What is the molarity of
the HNO3 solution?
2HNO3 + Ca(OH)2
Ca(NO3)2 + 2H₂O
Hint: Did
[?]M
Answers
The molarity of the HNO3 solution is 0.463 M.
To determine the molarity of the HNO3 solution, we can use the stoichiometry of the balanced chemical equation and the volume and molarity of the Ca(OH)2 solution used.
From the balanced equation, we can see that the stoichiometric ratio between HNO3 and Ca(OH)2 is 2:1. This means that for every 2 moles of HNO3, 1 mole of Ca(OH)2 is required to reach the equivalence point.
Given that the volume of the Ca(OH)2 solution used is 31.27 mL and its molarity is 0.167 M, we can calculate the number of moles of Ca(OH)2 used:
moles of Ca(OH)2 = volume (in liters) x molarity
= 0.03127 L x 0.167 mol/L
= 0.0052169 mol
Since the stoichiometric ratio is 2:1, the number of moles of HNO3 used is twice that of Ca(OH)2:
moles of HNO3 = 2 x moles of Ca(OH)2
= 2 x 0.0052169 mol
= 0.0104338 mol
Now we need to calculate the molarity of the HNO3 solution using the volume of the HNO3 solution used, which is 22.5 mL:
molarity of HNO3 = moles of HNO3 / volume (in liters)
= 0.0104338 mol / 0.0225 L= 0.463 M
for more such questions on molarity
https://brainly.com/question/30404105
#SPJ11
How many atoms of oxygen (o)are present 2naOH+H2SO4 2H2O+Na2SO4
Answers
Answer:
10
Explanation:
did i do this correctly?

Answers
The wavelength is 4 x 10¹²nm and the energy in joules is 2.65 x 10⁻¹⁹J, so yes.
How to calculate wavelength and energy?To answer the first question, use the equation:
wavelength = speed of light / frequency
where the speed of light is approximately 3.00 x 10⁸ m/s.
Substituting the given values:
wavelength = (3.00 x 10⁸ m/s) / (7.5 x 10₄ Hz) = 4000m
Therefore, the wavelength of the photon is (4000 m x 1 x 10⁹nm)/1m = 4,000,000,000,000nm²/1m = 4 x 10¹²nm.
To answer the second question, use the equation:
energy = Planck's constant x speed of light / wavelength
where Planck's constant is approximately 6.626 x 10⁻³⁴ Js.
Substituting the given values:
energy = (6.626 x 10⁻³⁴ Js) x (3.00 x 10⁸ m/s) / (750 x 10⁻⁹ m) = 2.65 x 10⁻¹⁹ J
Therefore, the energy of the photon is 2.65 x 10⁻¹⁹J.
Learn more on wavelength and energy here: https://brainly.com/question/27856390
#SPJ1
What is the general method/rule/formula for calculating where the ball will land?
Answers
The general method/rule/formula for calculating where a ball will land depends on various factors such as the initial velocity, angle of projection, air resistance, and gravity.
In general, the most common method to calculate the landing position of a projectile is to use the kinematic equations of motion, which relate the position, velocity, and acceleration of an object. These equations can be used to determine the time it takes for the ball to hit the ground and the horizontal distance it travels during that time.
However, the calculation can become more complex when considering factors like air resistance and varying conditions, and may require more advanced mathematical models.
Learn more about Resistance at
brainly.com/question/29427458
#SPJ1
Identify the substance that has formula mass of 133.5amu.
(a) MgCI
b)SCI
c)BCI
D) AICI
Answers
The calculated formula masses to 133.5 amu, we find that the substance with a formula mass closest to 133.5 amu is (d) AlCl3. Therefore, the answer is option D.
To identify the substance with a formula mass of 133.5 amu, we need to calculate the formula mass of each given substance and compare it to 133.5 amu.
(a) MgCl2:
The formula mass of MgCl2 can be calculated by adding the atomic masses of magnesium (Mg) and chlorine (Cl).
Mg: atomic mass = 24.31 amu
Cl: atomic mass = 35.45 amu
Formula mass of MgCl2 = (24.31 amu) + 2(35.45 amu) = 95.21 amu
(b) SCl:
The formula mass of SCl can be calculated by adding the atomic masses of sulfur (S) and chlorine (Cl).
S: atomic mass = 32.07 amu
Cl: atomic mass = 35.45 amu
Formula mass of SCl = 32.07 amu + 35.45 amu = 67.52 amu
(c) BCl:
The formula mass of BCl can be calculated by adding the atomic mass of boron (B) and chlorine (Cl).
B: atomic mass = 10.81 amu
Cl: atomic mass = 35.45 amu
Formula mass of BCl = 10.81 amu + 35.45 amu = 46.26 amu
(d) AlCl3:
The formula mass of AlCl3 can be calculated by adding the atomic mass of aluminum (Al) and 3 times the atomic mass of chlorine (Cl).
Al: atomic mass = 26.98 amu
Cl: atomic mass = 35.45 amu
Formula mass of AlCl3 = 26.98 amu + 3(35.45 amu) = 133.78 amu. Option D
For more such questions on masses visit:
https://brainly.com/question/24191825
#SPJ8
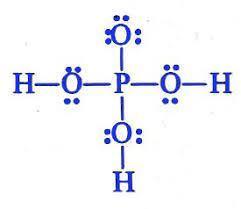
Which is the Lewis structure for H3PO4? An upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left. A central upper P is single bonded left, right, above, and below to upper Os. The O above the P is single bonded to upper H on the left and the right, and has two electron dots above it. The O below the P is single bonded to an H below, and has pairs of electron dots to the left and right. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below. A central upper P is bonded to an upper H above, an upper O below, and upper O's bonded to upper H's to the left and the right. The O below the P has three pairs of electron dots, to the left, right, and below; the other two O's have pairs of dots above and below. A central upper P is double bonded to an O above, and single-bonded to an upper O single-bonded to an upper H to the left and the right. The O above the P has three pairs of electron dots, to the left, above, and to the right; the O's to the right and left have pairs of dots above and below.
Answers
Answer:
It is A.
Explanation:
I took the test.
The Lewis structure shows the arrangement of valence electrons in H3PO4.
The Lewis structure gives us a picture of the number of valence electrons in a molecule. This is because, in a Lewis structure, the electrons in the molecule are shown as dots. A single line may be used to show shared electrons in a covalent bond.
The correct Lewis structure of H3PO4 is an upper P is single bonded above to an O, and to the left, right, and below to an O single bonded to an H. The O above the P has three pairs of dots to the left, above, and below; the O's to the sides have pairs of dots above and below, and the O below the P has pairs of dots right and left.
Learn more: https://brainly.com/question/4144781
List three things, other than the name, symbol, atomic number, and average atomic mass, you can discover about an element using the periodic table in Figure 6.9.
Answers
Answer: State of matter, Subcategory in the metal-metalloid-nonmetal trend, and electrons per shell.
Explanation:
How many moles of H2 are needed to produce 34.8 moles of NH3?
Answers
2 i hope this helps
:)✨✨✨✨✨✨
energy level of diagram
Answers
Answer:
The energy level diagram is used to represent the energy states available in each atom. When an electron is in an energy state, it emits nor absorbs radiation. A photon is emitted or absorbed when an electron transitions from one energy state to another.
Explanation:
If I have 5 mole of a gas at a pressure of 7.6 atm and a volume of 11 liters, what is the temperature?
Answers
Answer:
203.75 K
Explanation:
use the ideal gas law equation: PV=nRT, R being the constant 0.08206 L-atm/mol-K.
plug in the values given:
(7.6 atm) (11.0 L) = (5.0 mol) (0.08206 L-atm/mol-K) (T)
and solve for T!
Releases sugar (glucose) into the blood stream to power cells A. Brain B. Heart C. Liver D. Stomach and intestines
Answers
Answer:
C Liver
Explanation:
What is the density of a solid that has a mass of 9.2 grams and a volume of 12.3 cm3 ? Would this object float or sink in water?
Answers
\(\boxed{\sf Density=\dfrac{Mass}{Volume}}\)
\(\\ \sf\longmapsto Density=\dfrac{9.2}{12.3}\)
\(\\ \sf\longmapsto Density=0.74g/cm^3\)
Density of water =1g/cm^3It will float in water
Please please helppp

Answers
Answer:
is that geography because that is my favourite subject... I can help
Determine the volume (in mL) of 1.00 M NaOH that must be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
Answers
Approximately 70.57 mL of 1.00 M NaOH should be added to 250 mL of 0.50 M CH3CO₂H to produce a buffer with a pH of 4.50.
To determine the volume of 1.00 M NaOH required to produce a buffer with a pH of 4.50 when added to 250 mL of 0.50 M CH3CO₂H, we need to consider the Henderson-Hasselbalch equation and the stoichiometry of the reaction.
The Henderson-Hasselbalch equation for a buffer solution is given as:
pH = pKa + log([A-]/[HA])
In this case, CH3CO₂H (acetic acid) acts as the weak acid (HA) and CH3COO- (acetate ion) acts as its conjugate base (A-). We are given that the desired pH is 4.50, and we can determine the pKa value for acetic acid from reference sources, which is approximately 4.75.
Using the Henderson-Hasselbalch equation, we can rearrange it to solve for the ratio [A-]/[HA]:
[A-]/[HA] = 10^(pH - pKa)
[A-]/[HA] = 10^(4.50 - 4.75) = 10^(-0.25) = 0.5623
This means that the ratio of the acetate ion to acetic acid in the buffer solution should be approximately 0.5623.
To calculate the required volume of NaOH, we need to consider the stoichiometry of the reaction. Acetic acid reacts with hydroxide ions (OH-) to form acetate ions and water:
CH3CO₂H + OH- → CH3COO- + H2O
The stoichiometric ratio between acetic acid and hydroxide ions is 1:1. Therefore, the volume of 1.00 M NaOH needed can be calculated using the equation:
Volume (NaOH) × 1.00 M = Volume (CH3CO₂H) × 0.50 M × 0.5623
Volume (NaOH) = (Volume (CH3CO₂H) × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) = (250 mL × 0.50 M × 0.5623) / 1.00 M
Volume (NaOH) ≈ 70.57 mL
For more such questions on buffer visit:
https://brainly.com/question/13076037
#SPJ8
This table shows the relationship between the force on an object and the object's resulting acceleration.
Force vs. Acceleration
Force (N)
0
4
8
12
Acceleration
(m/s/s)
0
O 0.2 kg
O2 kg
O 0.5 kg
O 1 kg
2
4
6
What is the mass of the object?
HELP!!!!
Answers
2Kg is the mass of the object. Therefore, the correct option is option B among all the given options.
What is Acceleration?Acceleration is the rate at which velocity varies over time, both in terms of speed and direction. A point or object travelling in a straight path called accelerated if it accelerates or decelerates.
Regardless of whether the speed remains constant, movement on a circle becomes accelerated because of direction is always changing. Both redistribution to acceleration in all other types of motion.
F (N) Acceleration (m/s/s)
0 0
4 2
8 4
12 6
for the set 4,
F =ma
m = F/a
4/2=2
8/4= 2
12/6= 2Kg
Therefore, the correct option is option B.
To learn more about Acceleration, here:
https://brainly.com/question/24341162
#SPJ9
Your question is incomplete but most probably your full question was,
This table shows the relationship between the force on an object and the object's resulting acceleration.
F (N) Acceleration (m/s/s)
0 0
4 2
8 4
12 6
What is mass of the object?
A) 1Kg
B) 2Kg
C) 3Kg
D) 4Kg