What is the Mole fraction of Co2+ in a solution made up of 8 mL of 0.002 M solution of Co+ and 2 mL of 0.002 M solution of CN ?
Answers
The Mole fraction of Co2+ in a solution made up of 8 mL of 0.002 M solution of Co+ and 2 mL of 0.002 M solution of CN is 0.8.
The mole fraction is a unitless quantity that is defined as the number of moles of a substance divided by the total number of moles of all components in a solution. It is represented by the symbol "χ."The mole fraction of a solute in a solution can be determined by dividing the number of moles of the solute by the total number of moles of all components in the solution. In this case, the mole fraction of Co2+ can be calculated as follows: First, we need to calculate the number of moles of each component in the solution.
A number of moles of Co+:n = M × V = 0.002 M × 8 mL/1000 mL/L = 0.000016 molNumber of moles of CN-:n = M × V = 0.002 M × 2 mL/1000 mL/L = 0.000004 molThe total number of moles of all components in the solution are the sum of the number of moles of each component. Therefore, a Total number of moles:n = 0.000016 mol + 0.000004 mol = 0.00002 mol The mole fraction of Co2+ can now be calculated as:χ = n(Co2+)/n(total)= 0.000016 mol/0.00002 mol= 0.8Therefore, the mole fraction of Co2+ in the solution is 0.8.
Learn more about Mole fraction at https://brainly.com/question/31102608
#SPJ11
Related Questions
A gas sample with a volume of 1,500 cm® is heated from -65 °C to 75 °C. Assuming the
pressure remains constant, what is the volume of this sample, in cm", when the
temperature reaches 75 °C?
Answers
Answer:
V₂ = 2509.62 cm³
Explanation:
Given data:
Initial volume = 1500 cm³
Initial temperature = -65°C (-65 + 273 = 208 K)
Final temperature = 75°C ( 75 +273 = 348 K)
Final volume = ?
Solution:
The given problem will be solve through the Charles Law.
According to this law, The volume of given amount of a gas is directly proportional to its temperature at constant number of moles and pressure.
Mathematical expression:
V₁/T₁ = V₂/T₂
V₁ = Initial volume
T₁ = Initial temperature
V₂ = Final volume
T₂ = Final temperature
Now we will put the values in formula.
V₁/T₁ = V₂/T₂
V₂ = V₁T₂/T₁
V₂ = 1500 cm³ × 348 K / 208 k
V₂ = 522000 cm³.K / 208 k
V₂ = 2509.62 cm³
__AgNO3 + __Cu → __Ag + __Cu(NO3)2
Answers
The complete question is: Fill the blanks with appropriate coefficient number for the reaction equation- __AgNO3 + __Cu → __Ag + __Cu(NO3)2
Answer: The complete reaction equation is \(AgNO_{3} + Cu \rightarrow Ag + Cu(NO_{3})_{2}\)
Explanation:
A chemical equation which contains same number of atoms on both reactant and product side is called a balanced chemical equation.
For example, \(AgNO_{3} + Cu \rightarrow Ag + Cu(NO_{3})_{2}\)
Here, number of atoms present on reactant side is as follows.
Ag = 1\(NO_{3}\) = 1Cu = 1Number of atoms present on product side is as follows.
Ag = 1\(NO_{3}\) = 2Cu = 1To balance this equation, multiply \(AgNO_{3}\) by 2 on reactant side and multiply Ag by 2 on product side. Hence, the equation can be re-written as follows.
\(2AgNO_{3} + Cu \rightarrow 2Ag + Cu(NO_{3})_{2}\)
Now, the number of atoms present on reactant side are as follows.
Ag = 2\(NO_{3}\) = 2Cu = 1Number of atoms present on product side are as follows.
Ag = 2\(NO_{3}\) = 2Cu = 1Thus, we can conclude that the complete reaction equation is \(AgNO_{3} + Cu \rightarrow Ag + Cu(NO_{3})_{2}\).
true or false: Some fungi are unicellular and some fungi are multicellular
Answers
Answer:
True
Explanation:
Oceans, and other bodies of water, are found on which layer of Earth?
Outer core
Mantle
Inner core
Crust
Answers
they are found on the crust
Answer:
crust
Explanation:
Chemical Reactions - Problems
4 NH, +5 O, → 4 NO+6H_O
Which of the following are the reactants in the reaction above?
NH,
NO
I
H₂O
Submit v
IT
Answers
What is the mass of 14.4 moles Fluorine?
0.379g
274.8
1.32 g
2.64g
547 g
Answers
Answer:
D
Explanation: d
Answer:
547g
Hope i could help ;)
What is the name of the theory that proposes continents move over time due to the motion of the lithospheric plates?
Continental Slide
Convection Currents
Plate Drifting
Continental Drift
Answers
Answer:
plate drifting
Explanation:
Plate tectonics
Plate tectonics is the theory that Earth's land masses are in constant motion. The realization that Earth's land masses move was first proposed by Alfred Wegener, which he called continental drift.
Answer:
Plate drifting
Explanation:
Plate drifting is similar to plate tectonics.Earth's surface consists of different plates.These plates keep moving slowly all time .So slowly the continents have been formed by this tectonics (Still going on)What is the∆S° of 0₂
Answers
Answer:0
Explanation: zero because it is the most stable form of oxygen in its standard state
what happens when a gas obtained by heating Ammonium Chloride and slaked lime are passed through copper sulphate ?
Answers
Answer:
Consequently, what happens when gas obtained by heating slaked lime and ammonium chloride is passed through copper sulphate solution? The HCl in the gas mixture will form hydrochloric and the H+ will react with some of the NH3(aq), forming NH4^+, and with some of the SO4^2-, forming HSO4^-.
The gas obtained by heating Ammonium Chloride and slaked lime is passed through copper sulphate, deep blue solution will be formed.
Gas obtained from heating Ammonium ChlorideThe gas obtained by heating Ammonium Chloride and slaked lime is ammonia gas.
2NH₄Cl + Ca(OH)₂ ---> CaCl₂ + 2NH₃ + 2H₂O
Reaction of ammonia gas with copper sulphateAmmonia reacts with copper (II) ions to precipitate light blue copper hydroxide.
Ammonia causes the copper ion to go back into the solution as a deep blue ammonia complex.
However, in excess ammonia, the precipitate dissolves.
Learn more about ammonia gas here: https://brainly.com/question/7982628
#SPJ2
1 Write the correct symbol, with both superscript and subscript,
for each of the following. Use the list of elements in the front
inside cover as needed: (a) the isotope of platinum that contains
118 neutrons, (b) the isotope of krypton with mass number 84,
(c) the isotope of arsenic with mass number 75, (d) the isotope of
magnesium that has an equal number of protons and neutrons.
Answers
Answer:
+27 66 514 3800
Explanation:
Use the edit icon to pin, add or delete clips.
How many moles are in 16.94 g of H2O?
Answers
Answer:
0.9401 mol H₂O
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableUsing Dimensional AnalysisExplanation:
Step 1: Define
16.94 g H₂O
Step 2: Identify Conversions
Molar Mass of H - 1.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of H₂O - 2(1.01) + 16.00 = 18.02 g/mol
Step 3: Convert
\(16.94 \ g \ H_2O(\frac{1 \ mol \ H_2O}{18.02 \ g \ H_2O} )\) = 0.940067 mol H₂O
Step 4: Check
We are given 4 sig figs. Follow sig fig rules and round.
0.940067 mol H₂O ≈ 0.9401 mol H₂O
Can someone help me with my chemistry problem?

Answers
Answer:
D. 108g of water
Explanation:
16g CH4 produces 2(18)g of H20
1g CH4 produces \(\frac{36}{16}\)g of H2O
48g CH4 produces \(\frac{36}{16}\)×48
108g of H20
Answer:
D. 108g of water
Explanation:
buffer is prepared by adding 39.8 ml of 0.75 m naf to 38.9 ml of 0.28 m hf, ka = 6.8 10−4. what is the ph of the final solution?
Answers
The pH of the final solution is 3.09.
To solve this problem, we will use the Henderson-Hasselbalch equation, which relates the pH of a buffer solution to the pKa of the weak acid and the ratio of the concentrations of weak acid and its conjugate base;
pH = pKa + log([conjugate base]/[weak acid])
In this case, the weak acid is HF, and its conjugate base will be F⁻. The pKa of HF is given as 6.8 x 10⁻⁴. We are given the volumes and concentrations of the two solutions, so we can calculate the concentrations of HF and F⁻;
[HF] = 0.28 M x (38.9 ml / 78.7 ml) = 0.139 M
[F⁻] = 0.75 M x (39.8 ml / 78.7 ml) = 0.379 M
Now we can substitute these values into the Henderson-Hasselbalch equation;
pH = 6.8 x 10⁻⁴ + log(0.379/0.139)
= 3.09
Therefore, the pH of the final solution will be 3.09.
To know more about final solution here
https://brainly.com/question/23956784
#SPJ4
Which ion would you expect to have the largest crystal field splitting delta ? [Os(H2O)6]^2+ [Os(CN)6]^3 [Os(CN)6]^4- [Os( H2O)6]^3+
Answers
The ion expected to have the largest crystal field splitting delta is [Os(CN)6]^3-.
Crystal field splitting (delta) refers to the energy difference between the d-orbitals in a transition metal complex due to the interaction between the metal ion and the surrounding ligands. The magnitude of delta depends on the nature of the ligands, with stronger field ligands causing larger splitting.
In this case, we have two types of ligands: H2O (aqua) and CN- (cyanide). CN- is a stronger field ligand compared to H2O, as it has a higher electron-donating ability. Consequently, complexes containing CN- will have a larger crystal field splitting. Among the given complexes, [Os(CN)6]^3- has the highest oxidation state and is surrounded by the strong field CN- ligands, leading to the largest crystal field splitting delta.
To know more about crystal field visit:
brainly.com/question/29389010
#SPJ11
how is it d?explain please?! i do not understand.please dont guess
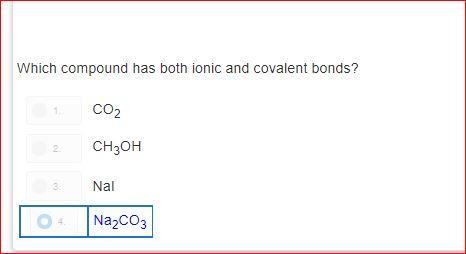
Answers
Answer:
As you can see sodium = ionic and covalent bonds aka NA 2
and CO 3 also has those two bonds the other answers dont have both numbers (bonds) Na2 and CO3 is the only answer choice that has these bonds in simpler terms ( only answer with 2 numbers)
Explanation:
____________ In a group all on their own. No neutrons.
____________ The groups containing the transition metals.
____________ The Lewis Dot Diagram for Bromine.
____________ The Bohr Diagram for Argon.
____________ 7 valence electrons, halogen family, 74 neutrons.
____________ 16 protons, neutrons, and electrons.
____________ Creator of the periodic table.
____________ Bohr diagram for oxygen.
____________ Period 4, transition metal, 26 electrons.
____________ Noble Gases.
____________ Lewis Dot Diagram for Phosphorus.
____________ 12 protons, neutrons, and electrons.
____________ Bohr diagram for Sulfur.
____________ Full outer shell, mass is less than 10, noble gas.
____________ Lewis dot diagram for Tin.
____________ The group of the Alkali Metals.
____________ Bohr diagram for Carbon.
____________ Alkaline Earth Metal, period 4.
____________ 13 protons, 13 electrons, 14 neutrons.
____________ Same atomic number, different atomic mass.
____________ Lewis dot diagram for Barium.
____________ 8 valence electrons, period 6.
____________ Bohr Diagram for Sodium.
____________ Lewis Diagram for Sodium.
____________ The rows of the periodic table.
____________ The columns of the periodic table.
i really need help these are the only ones i really need we have 10 min plss help
Answers
I need help!!! ASAP!!

Answers
Answer:
Yes it is balanced.
Explanation:
See 1.ferrous or iron is 3 on both sides.
2. Oxygen is also 4 on both side
3. Same in hydrogen (8 on both sides).
Which of the following compounds will most easily conduct electricity when dissolved in water?
CO2
O2
CCl4
MgF 2

Answers
Answer:
MgF₂
Explanation:
The correct answer is MgF₂, also known as magnesium fluoride.
This is because magnesium fluoride is an ionic compound, as there's a significant difference between the electronegativities of Mg and F.
Ionic compounds become good electricity conductors once they are dissolved in water.
Which is correct for the chromium isotope?

Answers
The most common isotope of chromium contains 28 neutrons in addition to its 24 protons and 24 electrons. Chromium is a strong, shiny metal with a bluish hue under normal circumstances. Thus, option C is correct.
What is chromium isotope?There are four naturally occurring isotopes of chromium, with relative abundances of 4.35%, 83.79%, 9.50%, and 2.36% for 50Cr, 52Cr, 53Cr, and 54Cr, respectively.
Numerous Chromium isotopes are employed in medical procedures. The radioisotope Cr-51, which is used to gauge blood volume and red blood cell survival, is created from Cr-50.
Studies on (adult) diabetes and chromium metabolism both use Cr-53 and Cr-54 as research tools.
Therefore, chromium isotope contain 24 protons and 28 neutrons.
Learn more about chromium isotope here:
https://brainly.com/question/681602
#SPJ1
Calculate the ratio of H* ions to OH-ions at a pH = 7.
Find the concentration of H* ions to OH ions listed in
Table B of your Student Guide. Then divide the H*
concentration by the OH concentration. Record this
calculated ratio in Table A of your Student Guide.
Compare your approximated and calculated ratios of H+
ons to OH-ions at a pH = 7. Are they the same? Why or
why not? Record your comparison in Table A.
What is the concentration of H+ ions at a pH = 7?
mol/L
What is the concentration of OH-ions at a pH = 7?
mol/L
What is the ratio of H+ ions to OH-ions at a pH = 7?
:
Please help!! Worth 100 points!!

Answers
a) The concentration of the hydrogen ions is 1 * 10^-7 M
b) The concentration of the hydroxide ions is 1 * 10^-7 M
c) Ratio of the hydrogen to the hydroxide ion is 1:1
What is the pH?We know that the pH of the solution would have to do with ratio of the hydrogen ion and the hydroxide ions that we have in the solution. We know that the pH values that range from 0 -6 is acid, 7 is neutral and 8 - 14 is basic.
Then we have that;
Concentration of the hydrogen ion in the solution is obtain from the formula;
[H^+] = Antilog (-pH)
At pH 7 we have;
[H^+] = Antilog (-7)
= 1 * 10^-7 M
Learn more about pH:https://brainly.com/question/15289714
#SPJ1
please help me with this question/

Answers
hshsbdndnensj
Answer:
4 of each protons and electrons
Explanation:
since the atomic number is 4 it would contain both the same number of protons and electrons
PLEASEEEE HELPPPP I’ll make you Brainlys Summary
Now, create of summary of what you've learned.
1. Complete the table below for protons, neutrons, and electrons. Respond with a Y for yes or N of no.
1
2
3
***
***
***
Question
Score: 0/4
Identifies Element?
Affects Mass?
Affects Charge?
***
Proton
***
Neutron
***
Electron
***

Answers
Answer:
Explanation:
For the first row, only protons identify an element. For the second row, protons and neutrons affect mass. For the third row, protons and electrons affect charge.
PLEASE HELP!!
How does one determine an empirical formula from a percent composition?
A. Convert mass percents to moles and then use the moles as
subscripts in the formula
B. Convert mass percents to grams and then divide by the smallest
gram value to find subscripts
C. Convert mass percents to moles and then divide by the smallest
mole value to get subscripts
D. Convert mass percents to grams and then divide by the smallest
mass percent value to find subscripts
Answers
B is incorrect because most likely, they would've already given you the mass in grams and it's just not how you find empirical formula
D & A is just wrong because the definition of empirical formula is the simplest, positive integer ratio of atoms present in a compound. Atoms, not grams.
What is the main point of the reading? Group of answer choices Many elements combine to make a few compounds. Hawaii is made up of only a few types of compounds A few elements combine to make many compounds. Elements cannot be combined except by artificial means.
Answers
Answer:
A few elements combine to make many compounds.
Explanation:
The heading of the reading is 'Few elements, many Compounds'. The reading has focused on the process involved in the formation of elements and compounds. In the Hawaiian Islands the elements are found in abundance. These elements combine together to form compounds. The mountains, greenery, rocks and living matter are made up of many elements and compounds. The elements present helps in forming various compounds.
describe the main idea of this lab. in other words, what was the message this lab conveys about pollution?
Answers
Pollution refers the release of hazardous pollutants into the environment. These hazardous elements are referred to as contaminants. Pollutants may be naturally occurring, such as volcanic ash.
They may also be caused by human activities, such as factory runoff or waste. Pollutants have a negative impact on the quality of the air, water, as well as land.
Pollution refers the release of hazardous pollutants into the environment. These hazardous elements are referred to as contaminants. Pollutants may be naturally occurring, such as volcanic ash. They may also be caused by human activities, such as factory runoff or waste. Pollutants have a negative impact on the quality of both the air, water, as well as land.
To know more about Pollution
https://brainly.com/question/28519286
#SPJ4
How many grams of H2O will be produced if 750. grams of Fe are produced?
Fe3O4 + 4H2 - 3Fe + 4H20
Answers
Answer:
\(\boxed{\small \sf \: Mass \: of \: H_2O =322.2 \: grams}\)
Explanation:
Given:
Mass of ferous (Fe) produced = 750 gram.
To find:
Mass of water produced= ?
Solution:
Molar mass of Fe is 55.84 gram/mol
Let's find out the number of moles of ferous produced.
\( \small \sf Number \: of \: moles = \frac{Given \: mass \: of \: substance }{Molar \: mass \: of \: substance} \)
Substituting the given data in above formula.
\( \small \sf Number \: of \: moles = \frac{750}{55.84} \)
\( \boxed{\small \sf Number \: of \: moles \: of \: Fe= 13.43 \: moles}\)
Now the given reaction is,
\(Fe_3O_4 + 4H_2 \rightarrow 3Fe + 4H_2O\)
For every 3 mole of production of Fe, 4 mole of waters are produced. let for 13.43 moles of Fe x moles of H2O will be produced.now calculate the number of moles of H2O
\( \sf \: \frac{3}{4} = \frac{13.43}{x} \\ \sf x = \frac{13.43 \times 4}{3} \\ \sf x = 17.90 \: moles\)
\( \small \boxed{\sf number \: of \: moles \: of \: H_2O= 17.90 \: moles}\)
mass of one mole of H2O is 18 gram, so we can calculate mass of 17.90 moles.
\(\small \sf \: Mass \: of \: H_2O = 17.90 \times 18 \\ \boxed{\small \sf \: Mass \: of \: H_2O =322.2 \: grams}\)
Thanks for joining brainly community!
Use the standard reduction potentials in the appendix to calculate the standard free-energy change deltaG^degree, and the equilibrium constant, K, at 298 K for the reaction
4Ag(s) + O2(g) + 4 H^+ (aq) yields 4 Ag+(aq) + 2H2O(l)
Answers
The standard free energy change and the equilibrium constant is -1.75 x 10^5 J/mol and 6.67 x 10^31 respectively for the reaction.
We can calculate standard free-energy change ΔG° by using the following formula :
ΔG° = -nFE°
where ΔG° is the standard free energy change, n is the number of electrons transferred during the reaction, F is the Faraday constant which is (96,485 C/mol), and E° is the standard reduction potential.
Firstly we can split the equations in two halves to calculate the standard reduction potential of each equation
Ag(s) → Ag+(aq) + e- E° = +0.80 V
O2(g) + 4 H+(aq) + 4 e- → 2 H2O(l) E° = +1.23 V
The net reaction is the sum of both the standard reduction potential of each equation
4 Ag(s) + O2(g) + 4 H+(aq) → 4 Ag+(aq) + 2 H2O(l)
Applying the formula
ΔG° = -nFE°
ΔG° = -(4)(96,485 C/mol)(+0.80 V + 1.23 V)
ΔG° = -1.75 x 10^5 J/mol
Therefore the standard free-energy change ΔG° = -1.75 x 10^5 J/mol
To calculate the equilibrium constant, K, we can use the value from the standard free energy change using the following equation:
ΔG° = -RT ln K
in which R is the gas constant (8.314 J/mol·K), T is the temperature in Kelvin (298 K), and ln is the natural logarithm and the value of ΔG° from above
Applying the formula we get:
-1.75 x 10^5 J/mol = -(8.314 J/mol·K)(298 K) ln K
ln K = 72.99
K = e^72.99
K = 6.67 x 10^31
Therefore, the equilibrium constant for the reaction at 298 K is K = 6.67 x 10^31.
To learn more about standard free-energy change ,
https://brainly.com/question/11104816
#SPJ4
Combustion A gaseous hydrocarbon fuel (CxH2x+2) is combusted with air in an industrial furnace. Both the fuel and air enter the furnace at 25°C while the products of combustion exit the furnace at 227°C. The volumetric analysis of the products of combustion is: Carbon dioxide (CO₂) 9.45% Carbon monoxide (CO) 2.36% Oxygen (O₂) 4.88% Nitrogen (N₂) 83.31% Write a balanced chemical equation for the combustion reaction (per kmol of fuel) and hence determine the fuel and the air-fuel ratio. Construct separate 'reactants' and 'products' tables giving the number of moles and molar enthalpies for each of the reactants and products, respectively, involved in the combustion process. Hence determine the heat transfer rate and the combustion efficiency on a lower heating value (LHV) basis.
Answers
The balanced chemical equation for the combustion reaction of the gaseous hydrocarbon fuel (CxH2x+2) with air can be written as CxH2x+2 + (2x + 1)O2 + 3.76N2 -> xCO2 + (x + 1)H2O + 3.76(2x + 1)N2. The fuel is determined to be methane (CH4).
The balanced chemical equation for the combustion reaction of the gaseous hydrocarbon fuel (CxH2x+2) with air can be written as:
CxH2x+2 + (2x + 1)O2 + 3.76N2 -> xCO2 + (x + 1)H2O + 3.76(2x + 1)N2.
Given the volumetric analysis of the products of combustion, we can determine the value of x in the hydrocarbon fuel. The percentage of carbon dioxide (CO2) corresponds to the carbon atoms in the fuel, so 9.45% CO2 implies x = 1. The fuel is therefore methane (CH4).
To calculate the air-fuel ratio, we compare the moles of air to the moles of fuel in the balanced equation. From the equation, we have (2x + 1) moles of oxygen (O2) and 3.76(2x + 1) moles of nitrogen (N2) for every 1 mole of fuel. Substituting x = 1, we find that the air-fuel ratio is 17.2 kg of air per kg of fuel.
To determine the heat transfer rate and combustion efficiency on a lower heating value (LHV) basis, we need to calculate the molar enthalpies of the reactants and products. Using standard molar enthalpies of formation, we can calculate the change in molar enthalpy for the combustion reaction. The heat transfer rate can be obtained by multiplying the change in molar enthalpy by the mass flow rate of the fuel. The combustion efficiency on an LHV basis can be calculated by dividing the actual heat transfer rate by the ideal heat transfer rate.
Learn more about hydrocarbon : brainly.com/question/30666184
#SPJ11
Describe the main energy level shape and of the 2p * orbital
Answers
Answer:
The letters, s, p, d, and f designate the shape of the orbital. (The shape is a consequence of the magnitude of the electron's angular momentum, resulting from its angular motion.) An s orbital is spherical with its centre at the nucleus.
what effect will the addition of a small amount of strong acid have on a buffer solution? what effect will the addition of a small amount of strong acid have on a buffer solution? no effect drastically lowers the ph slightly lowers the ph slightly raises the ph drastically raises the ph
Answers
There will be no effect of adding a small amount of strong acid to a buffer solution.
The conjugate base in the buffer eats the hydronium ion, turning it into water and the weak acid of the conjugate base when a strong acid (H3O+) is added to the buffer solution. This causes the amount of weak acid to increase and the amount of conjugate base to decrease.
A buffer solution is an aqueous mixture of a weak acid and either its conjugate base or base itself. When a modest amount of a strong acid or base is applied to it, the pH hardly changes at all.
A buffer is a substance that can withstand a pH change when acidic or basic substances are added.
For more information on buffer solutions kindly visit to
https://brainly.com/question/22821585
#SPJ4