what is the pH of a saturated solution of cobalt hydroxide,Co(OH)2, Ksp=5.92x10^-15?
A.9.057
B. 9.358
C. 4.542
D. 6.886
E. 9.157
Answers
The pH of a saturated solution of cobalt hydroxide, Co(OH)², with a Ksp of 5.92x10⁻¹⁵ is approximately 9.354, which is closest to option 9.358. Therefore, the correct option is B.
This can be determined by following these steps:
Step 1: Write the dissociation equation for cobalt hydroxide.
Co(OH)₂ (s) ⇌ Co²+ (aq) + 2OH- (aq)
Step 2: Write the expression for Ksp.
Ksp = [Co²+] [OH-]²
Step 3: Let the concentration of Co₂+ be x, then the concentration of OH- will be 2x (since 2 moles of OH- are produced for every mole of Co(OH)₂ that dissolves).
Ksp = (x)(2x)²
Step 4: Plug in the value of Ksp and solve for x.
5.92x10⁻¹⁵ = x(2x)²
5.92x10⁻¹⁵ = 4x₃
x = (5.92x10⁻¹⁵ / 4)^(1/3)
x ≈ 1.13x10⁻⁵
Step 5: Calculate the concentration of OH- ions.
[OH-] = 2x ≈ 2.26x10⁻⁵
Step 6: Calculate the pOH using the formula:
pOH = -log[OH-]
pOH ≈ -log(2.26x10⁻⁵) ≈ 4.646
Step 7: Calculate the pH using the relationship between pH and pOH.
pH + pOH = 14
pH = 14 - pOH ≈ 14 - 4.646 ≈ 9.354
The pH of the saturated solution of cobalt hydroxide is approximately 9.354, which is closest to option B, 9.358.
To know more about saturated solutions refer here:
https://brainly.com/question/30599715#
#SPJ11
Related Questions
Which of the following best describes homeostasis?
A: The ability to maintain a reasonably stable internal condition regardless of surrounding factors.
B: The ability to react to threats by spiking heart rate and blood pressure. C: The ability to maintain a relatively stable external environment.
D: The ability to convert nutrients into energy.
Answers
Answer:
A: The ability to maintain a reasonably stable internal condition regardless of surrounding factors.
Explanation:
ex: Humans
hope this helps you
4. Manik saw his father watering his garden plants in hot weather. He noticed that
water doesn’t stick to the plant leaves and leaves become dry but looked fresh. He asked
following questions to his teacher
a. Which tissue forms the outer covering of a plant and does it have a protective role
to play?How ?
b. Why does water not stick to the leaves?
Answers
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
What tissues protects the leaves?We know that the leaves are the parts of the plant that are involved in photosynthesis. Photosynthesis is the process by which green plants produce their own food in the presence of sunlight and chlorophyll. We know that the leave has an outer protective covering.
The tissue that plays this outer covering of a plant for is the epidermis and its waxy cuticle. It prevents damage to the plant.
Water does not stick to the leaves of the plant owing to the fact that the leaves has a waterproof cuticle.
Learn more about leaves:https://brainly.com/question/12539285
#SPJ1
45. The following data was collected for 3 compounds:
Mass of Nitrogen that combines with 1 g of Oxygen
Compound A 1.750 g
Compound B 0.8750 g
Compound C 0.4375 g
Show whether these are the same or different compounds. What chemical law is being observed here?
Answers
Answer:
The three compounds are different compounds
Explanation:
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound A = 1.750 g
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound B = 0.8750 g
The mass of Nitrogen that combines with 1 gram of Oxygen in Compound C = 0.4375 g
According to the law of multiple proportions, when atoms of two different elements react to form compounds, the masses of one of the elements that combines with a fixed mass of the other element are in small whole number ratios.
The ratio of the masses are;
Mass of Nitrogen in Compound B/(Mass of Nitrogen in Compound C = 0.8750/0.4375 = 2
Mass of Nitrogen in Compound A/(Mass of Nitrogen in Compound C = 1.750/0.4375= 4
Mass of Nitrogen in Compound A/(Mass of Nitrogen in Compound B = 1.750/0.8750= 2
Given that the masses of Nitrogen in the three compounds are in small whole number ratios, the three compounds, Compound A, Compound B, and Compound C are different compounds.
what part of body birds use to attack
Answers
Answer:beek
Explanation:
Explain why the addition of a catalyst can make a reaction occur that doesn't normally occur at room temperature even when AG<0.
Answers
A catalyst can cause a reaction to occur that does not normally happen at room temperature by reducing the activation energy needed to undergo the reaction.
What is a catalyst?Catalysts are substances, often enzymes, used to speed up or commence a chemical reaction. They do this by reducing the necessary energy amount needed to begin the reaction, known as activation energy, or simply by changing the way in which the reaction takes place, which can allow for a reaction to take place even at room temperature when it would otherwise not occur.
Therefore, we can confirm that a catalyst can cause a reaction to occur that does not normally happen at room temperature by reducing the activation energy needed to undergo the reaction.
To learn more about catalysts visit:
https://brainly.com/question/12260131?referrer=searchResults
Millions of years ago the Earth formed as a giant ball of molten rock. The outer surface
cooled forming a thin, solid outer crust. Volcanic activity on the surface produced an
atmosphere containing the compounds carbon dioxide, ammonia, methane and water
vapour.
Describe the bonding in any one of these compounds. You must include electronic
structures in your explanation.
Answers
The chemical bond between carbon and oxygen to form carbon dioxide involves sharing of electrons between the atoms and is known as a covalent bond.
What is chemical bonding?A chemical bond is a link that holds two or more atoms together.
An enduring attraction between atoms or ions known as a chemical bond is what allows molecules and crystals to form. The bond may be created by the sharing of electrons in covalent bonds or by the electrostatic attraction of two oppositely charged ions, as in ionic bonds.
Covalent bonds are formed between electronegative elements that share electrons. These are usually atoms of the same or different non-metals. For example, the bond between carbon and oxygen to form carbon dioxide is a covalent bond.
Ionic bonds are formed between atoms of an electropositive element and atoms of an electronegative element. These are usually between metals ad non-metals.
Learn more about chemical bonds at: https://brainly.com/question/1974529
#SPJ1
work from Burning fuel is kinetic energy or potential energy
Answers
The energy stored in gasoline is released by burning it (combustion). During combustion, chemical bonds are broken and reformed (changing gasoline into byproducts such as water and carbon dioxide) releasing energy. There are many examples of chemical potential energy being converted to kinetic energy to do work.
a sample of gas weighs 3.33 g and occupies a volume of 1.365 l at 95 °c and 790 torr. identify the gas sample.A. Cl₂ (molar mass-70.90 g/mol)B. NH (molar mass- 17.03 g/mol)C. N₂0 molar mass-44.02 g/mol)D. CHC, (molar mass-119.4 g/mol)E. SO₂ (molar mass - 64.07 g/mol)
Answers
The gas sample is Cl₂. Answer A.
The ideal gas equation formula PV = nRT
P = the gas pressure (atm)1 atm = 760 torr
P = 790 torr = 790 ÷ 760 = 1.04 atmV = the gas volume (L)
V = 1.365 Ln = the number of moles (mol)R = the gas constant = 0.0821 L atm/K molT = the temperature (K)
T = 95 °C = 95 + 273 = 368 K
Calculating the number of moles of the gas sample.
PV = nRT
1.04 × 1.365 = n × 0.0821 × 368
1.4196 = n × 30.21
n = 1.4196 ÷ 30.21
n = 0.04699 mol
The formula for mass and number of moles m = n × Mr
m = the mass of the gas (grams)m = 3.33 gMr = the molar mass (g/mol)
A. Mr Cl₂ = 70.90 g/mol
B. Mr NH₃ = 17.03 g/mol
C. Mr N₂O = 44.02 g/mol
D. Mr CHCl₃ = 119.4 g/mol
E. Mr SO₂ = 64.07 g/mol
Calculating the molar gas from the sample
Mr = m ÷ n
Mr = 3.33 ÷ 0.04699
Mr = 70.9 g/mol
From the info given, gas Cl₂ has the same molar mass as the sample.
So, the gas sample is Cl₂, chlorine gas.
Learn more about ideal gas equation here: https://brainly.com/question/20348074
#SPJ4
NEED HELP ASAP GIVING 20 POINTS AND BRAINLIEST
Given 18.5 grams of CHA and 24.0 grams of Oz the following reaction occurs. Calculate the number of grams of
carbon dioxide that form from the reaction.
CH4 + 20 + CO2 + 2 H2O
Answers
Answer:
CO2 should be 50.88 g
Explanation:
CH4 + 2 O2 --> CO2 + 2 H2O
First, find the moles of CH4:
Molar Mass of CH4 (formular mass):
12+1*4=16 g/mol (actually just use the atomic mass in your periodic table to add them up)
Then we can find how many moles in CH4 = 18.5÷16=1.15625 mol
As we know 1 mole of methane(CH4) makes 1 mole of CO2, so if 1.15625 moles of methane is reacted, 1.15625 moles of CO2 will be made.
So the moles of CO2 is 1.15625 moles.
Calculate the mass of CO2:
Molecular mass: 12+16*2=44 g/mol
Mass=44*1.15625=50.88 g (cor. to 2 d.p.)
HURRY
What is the volume of a cube with an edge length of 0.843
Answers
Answer:
5.004
Explanation:
............
............
.my Rude g ubddvbrbrbr
sorry in a hurry
Answer: 0.6
Explanation:Yes
What is the relationship between a white dwarf and black dwarf?
Answers
Answer:
No longer emitting heat or light, the white dwarf will become a black dwarf. Because it emits no radiation, it is nearly impossible to see. However, the black dwarf would still retain its mass, allowing scientists to detect the effects produced by its gravitational field.
Explanation:
Answer:
white dwarfs are hot, black dwarfs are cold
Explanation:
a white dwarf is a slowly burning and aging corpse of a main sequence star such as our sun. they are up to 40 times hotter then the sun, ranking among one of the hottest objects in the universe, except their luminosity is so low that all their heat stays in them basically forever.
On the other hand, a black dwarf is a theoretical ending to a white dwarf, over the course of trillions of years, white dwarfs cool down into black dwarfs. black dwarfs rank among the coldest possible temperature. We aren't sure if these are really a thing, because we still haven't given white dwarfs enough time to cool down.
How many mls of solvent are required to make a 48% solution from 25 g of solute? (round to the nearest tenth with no units!)
Answers
To make a 48% solution from 25 g of solute, you would need approximately 52.08 mL of solvent.
To calculate the volume of solvent required, we need to consider the mass percent of the solution. The mass percent is defined as the ratio of the mass of solute to the total mass of the solution, multiplied by 100. In this case, the mass percent is given as 48%.
To find the volume of solvent, we can set up a proportion using the mass percent. Let's assume the total volume of the solution is V mL. We can set up the following equation:
(25 g)/(V mL) = (48 g)/(100 mL)
Cross-multiplying and solving for V, we get:
25V = 48 * 100
V = (48 * 100)/25
V ≈ 192 mL
Therefore, you would need approximately 192 mL of the solvent to make a 48% solution from 25 g of solute.
for such more questions on solution
https://brainly.com/question/25326161
#SPJ8
theoretical yield of Li2O
Answers
what is the activation energy (in kj) of a reaction whose rate constant increases by a factor of 100 upon increasing the temperature from 300 k to 360 k?
Answers
The activation energy of a reaction is ~ 69KJ/mol, at the given temperature.
What is Activation energy?The smallest additional amount of energy that a reactive molecule needs in order to transform into a product is known as activation energy. The minimal amount of energy required to activate or energize molecules or atoms so that they can engage in a chemical reaction or transformation is another way to put it.
What is Temperature?
The concept of temperature is used to convey quantitatively how hot and cold something is. Using a thermometer, one can measure, temperature.
Calculations:
Given,
K2 = 100K1
T1 = 300
T2 = 360
Now,
We know that,
ln K2/K1 = Ea/R * {(1/T1) - (1/T2)}
ln 100K1/K1 = Ea/R * {(1/300) - (1/360)}
Ea= ln 100K1/K1 * R/{(1/300) - (1/360)}
Ea ~ 69KJ/mol.
Hence, the activation energy of a reaction is ~ 69KJ/mol, at the given temperature.
To know more about Activation energy, check out:
https://brainly.com/question/26724488
#SPJ4
what is an example of an atomic number
Answers
The circled number in red is the atomic number.
(The site I used for this example is ptable.com. Very useful periodic table!)

Answer: in a sodium atom, there are 11 electrons and 11 protons. Thus the atomic number of Na atom = number of electrons = number of protons = 11.
Explanation:
which is the correct answer ?
Protein-, peptide-, and amine-based hormones have which of the following in common? amino acids gluccose O Lipids and steroids Cholesterol
Answers
Protein, peptide and amine-based hormones have amino acids in common. Amino acids are the building blocks of protein. The synthesis of these hormones takes place in the endocrine glands which are responsible for producing and releasing hormones into the bloodstream.
The amino acids which make up these hormones, are linked together to form a long chain of amino acids, which can be broken down into smaller peptides. These peptides are then modified in various ways to produce the final hormone. The modification can include the addition of carbohydrates, lipids, and other molecules.
The hormones are then transported through the bloodstream to their target cells where they bind to specific receptors on the surface of the cell. Once bound, the hormone can either activate or inhibit certain cellular functions which results in a specific physiological response.In conclusion, the correct answer is that protein-, peptide-, and amine-based hormones have amino acids in common.
To know more about hormones visit:-
https://brainly.com/question/30367679
#SPJ11
A tank initially holds 200 gal of brine solution containing 3lb of salt. At t=0, another brine solution containing 3lb of salt per gallon is poured into the tank at the rate of 6gal/min, while the well stirred mixture leaves the tank at the same rate. Find the time at which the mixture in the tank contains 6lb of salt. 0.216min 0.168min 2.13min 0.02min
Answers
The tank contains 200 gallons of brine solution with 3 lb of salt. At t = 0, another solution is poured at 6 gal/min, leaving the tank at the same rate. The incoming rate of salt is 18 lb/gal, and the outgoing rate is 6 gal/min. The solution's volume remains constant.
Given that,Initially, the tank holds 200 gallons of brine solution containing 3 lb of salt.At t = 0, another brine solution containing 3 lb of salt per gallon is poured into the tank at the rate of 6 gal/min.The mixture leaves the tank at the same rate.The volume of the tank remains constant.
Now, let's assume that x(t) be the amount of salt in the tank at time t.Therefore,x(0) = 3 lb.
Now, let's find the differential equation that x(t) follows.
In the tank, the incoming rate of salt = 3 lb/gal x 6 gal/min = 18 lb/min.The outgoing rate is 6 gal/min.
The volume of the tank is constant, and it is equal to 200 gallons.∴ (d/dt)x(t) = (18 - 6x(t)/200) lb/min = (9 - 3x(t)/100) lb/min (On dividing by 2)Separating the variables and integrating, we get:
∫(1/9 - x/300) dx = ∫dt Let u = x/300,
then du = (dx/300)Hence,
∫(1/9 - x/300) dx = ∫\(dt(u/2 - u^2/2)\) = t + C [Where C is the constant of integration]
x/600 - x^2/600 = t + C
Now, we need to find the value of C.
\(C = x(0)/600 - x(0)^2/600C\)
3/600 - 9/360000C = -1/2000
Hence,
\(x/600 - x^2/600 = t - 1/2000\)
Multiplying by 600 on both sides, we get,
\(x - x^2 = 600t - 0.3\)...(1)
Now, we need to find the value of t at which the mixture in the tank contains 6 lb of salt.Substituting x = 6 in equation (1), we get:6 - 6^2 = 600t - 0.3...5 = 600t...t = 5/600 = 0.00833 hours = 0.5 minutes
Therefore, the time at which the mixture in the tank contains 6 lb of salt is 0.5 minutes (approximately).Hence, the correct option is 0.02 min.
To know more about solution Visit:
https://brainly.com/question/15757469
#SPJ11
From where in the solar system did scientists conduct their spectral analyses in
1948? How do you know?

Answers
Answer:
Both Earth and Space
Explanation:
Because in 1948 there was no ability to analyze from space but in 1966 there was.
What is the relationship between the mass difference and the difference in
percent solution?
Answers
Relationship between the mass difference and the difference in percent solution is the mass percent give the ratio between the mass of a component in a mixture and the total mass of the mixture
Percent by mass is in term of the masses of the solute and the solution and it is ratio of the mass of solute to the total mass of the solution and percent by volume on the other hand is in term of the volume of the solute and the solution and the difference between mass percent and percent composition is that mass percent give ratio between the mass of a component in a mixture and total mass of the mixture
Know more about percent solution
https://brainly.com/question/6992535
#SPJ1
24. What is the difference between a Polyatomic Ion and a Compound?
Answers
The difference between polyatomic ions and a compound is that polyatomic ions have positive or negative charge, while compounds have no net electrical charge.
thiols are strong-smelling compounds responsible for
Answers
Answer:
Many thiols have pungent scents that resemble garlic or rotting eggs. Thiols are utilized as odorants to aid in the detection of natural gas (which is odorless in its pure state), and the "fragrance of natural gas" is owing to the thiol's smell.
Explanation:
What is the name given to the process shown in the diagram
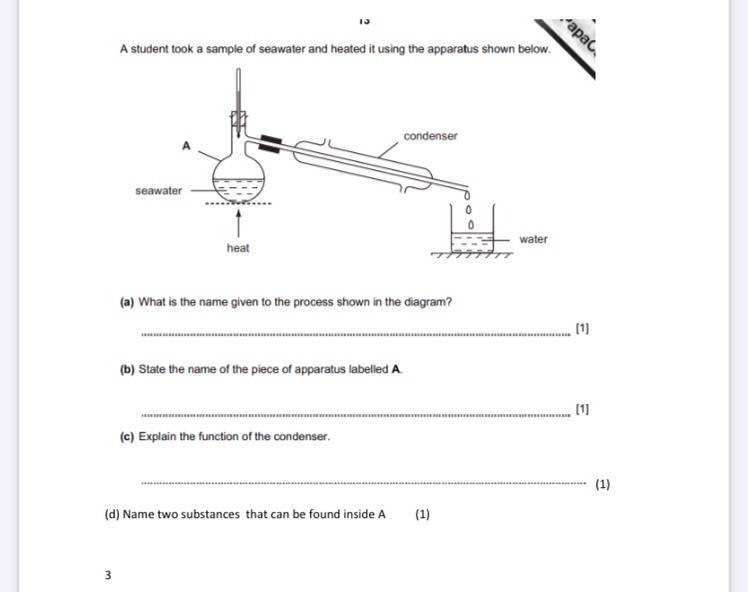
Answers
What type of stress results when two plates converge? Compression Shear Hot spot
Answers
Answer:
Compression
Explanation:
Hi! When two convergent plates collide, they should create compressive pressure, or compression.
An insoluble solid that forms from a chemical reaction is called
Answers
Precipitates are insoluble ionic solid products of a reaction, formed when certain cations and anions combine in an aqueous solution. The determining factors of the formation of a precipitate can vary.
Calculate the densities of the objects using the volumes found by displacement. Record your data
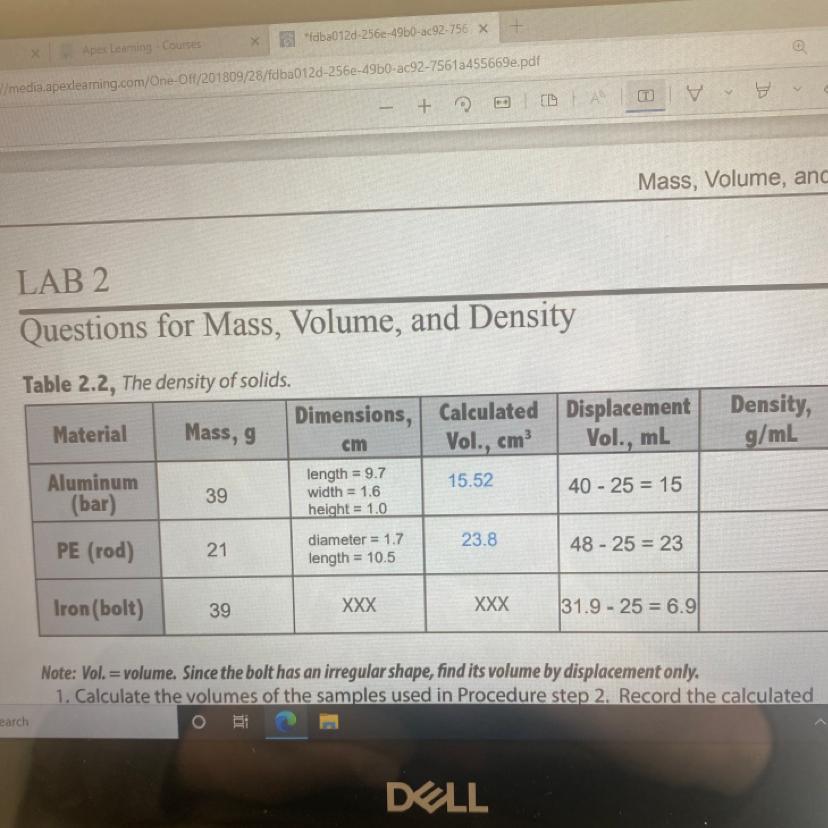
Answers
The densities of the substances are;
Aluminum bar = 2.6 g/ml
PE rod = 0.91 g/mL
Iron bolt = 5.65 g/mL
What is density?The term density is defined as the ratio of the mass to the volume of the object. We know that mass is an intrinsic property. This means that the density of the object can be used to identify what the object that is under study is. Let us now try to find the density of each of the objects.
In this case, we have the mass and the volumes of the objects as they have been shown in the table that have here. We can now be able to find the volume of each of the objects.
Density of aluminum bar = 39 g/ 15 g/mL = 2.6 g/ml
Density of the PE rod = 21 g/23 mL = 0.91 g/mL
Density of iron bolt = 39 g/6.9 mL = 5.65 g/mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
What pillar of sustainability is broken by recycling
electronics in India? Should the US make a law that electronics can
only be recycled in the US?
Answers
The pillar of sustainability broken by recycling electronics in India is environmental sustainability. Implementing a law that restricts electronics recycling to the US would not necessarily be the most effective solution, as it overlooks the complex global dynamics of electronic waste management.
Recycling electronics in India often involves improper disposal methods, such as burning or dismantling without proper safety measures. This leads to environmental pollution, including the release of hazardous substances into the air, soil, and water, thus violating the principle of environmental sustainability.
However, simply mandating that electronics can only be recycled in the US may not be the most optimal solution. Electronic waste is a global issue, and restricting recycling to a single country disregards the fact that electronic products are manufactured and consumed worldwide. A more comprehensive approach to addressing electronic waste would involve international cooperation, strict regulations, and monitoring of recycling practices to ensure they meet environmental standards.
Efforts should focus on improving recycling practices globally, including promoting responsible electronic waste management, developing sustainable recycling infrastructure in multiple countries, and encouraging the adoption of safe and environmentally friendly recycling practices. This approach would foster global sustainability and address the challenges associated with electronic waste disposal more effectively than a geographically limited restriction.
To learn more about sustainability, here
https://brainly.com/question/32771548
#SPJ4
Is salt water solution a homogeneous mixture
Answers
Answer:
yes, it is a homogeneous mixture because the different parts cannot be seen.
Explanation:
Which of the following elements does not form a diatomic molecule?
A. Chlorine.
B. Nitrogen.
C. Neon.
D. Hydrogen.
Answers
Answer:
C
Explanation:
Neon is a noble gas, which is monoatomic.
which of the following accurately describes the ph scale? which of the following accurately describes the ph scale? the ph scale runs from 0 (neutral) to 14 (most acidic), with 7 as an average acidity level. the ph scale runs from 0 (most acidic) to 14 (neutral), with 7 as an average acidity level. the ph scale runs from 0 (most basic) to 14 (most acidic), with 7 as a neutral. the ph scale runs from 0 (most acidic) to 14 (most basic), with 7 as a neutral.
Answers
Answer:
The pH scale measures acidity of a substance. known as potential of hydrogen, it varies from 0 to 14 with 7 being the pH value of a neutral solution. Below 7 shows the substance is acidic in nature and above 7 is alkaline in nature. pH 0-3 are considered strong acids while pH 4-6 are weak acids. pH 8-10 are weak alkalines and pH 11-14 are strong alkalines. This is a general trend and there may be exeptions especially if the substance has a negative pH. However, it would not be covered likely unless you are doing university chemistry.
why are carcinogens a concern to people?
Answers
Answer:
Bcoz the carcinogenes damage the genome or to the disruption of cellular metabolic processes
Explanation:
Answer:
Carcinogens may increase the risk of cancer by altering cellular metabolism or damaging DNA directly in cells, which interferes with biological processes, and induces the uncontrolled, malignant division, ultimately leading to the formation of tumors.
Explanation: