What is the reagent used to oxidize a primary alcohol into an aldehyde?
Answers
The reagent commonly used to oxidize a primary alcohol into an aldehyde is called pyridinium chlorochromate (PCC).
Pyridinium chlorochromate (PCC) is a mild oxidizing agent that selectively converts primary alcohols into aldehydes without further oxidation to carboxylic acids. This is due to the fact that PCC is a relatively weak oxidizing agent compared to other reagents such as potassium permanganate or chromic acid, which would fully oxidize the aldehyde into a carboxylic acid.
The reaction between PCC and a primary alcohol occurs via a hydride transfer mechanism. PCC acts as an electrophile, accepting hydride ions from the alcohol and transferring them to the chromium center, which is reduced in the process. This forms a chromate ester intermediate, which then undergoes hydrolysis to yield the aldehyde product.
Overall, the use of PCC as an oxidizing agent for primary alcohols provides a useful synthetic tool for the selective production of aldehydes, which are important intermediates in many organic synthesis reactions.
Learn more about Pyridinium chlorochromate (PCC) here: https://brainly.com/question/30695312
#SPJ11
Related Questions
The presence of which ion usually produces a colored solution? A. K+
B. F-
C. Fe2+
D. S2-
Answers
The presence of which Fe²⁺ ion usually produces a colored solution. Therefore, option C is correct.
What is an ion?An ion can be described as a chemical specie that exhibited a positive or negative charge. The term ‘ion’ can be referred to atoms or molecules with non-zero charges on them. Therefore, all atoms have more electrons than protons or more protons than electrons.
If the number of protons in atomic structure is greater than electrons are termed to hold a net positive charge and are referred to as cations. The ions that contain a greater number of electrons than protons in atomic structure hold a net negative charge are anions.
The electronic configuration of the iron is [Ar]3d⁶4s². As the iron atom losses 2 electrons in its valence shell, it becomes a cation Fe²⁺ ion. As it has four unpaired electrons produces a colored solution.
Learn more about an ion, here:
brainly.com/question/13692734
#SPJ2
a. How many protons?
b. How many neutrons?
c. What is the name of this atom?
PLS HELP ILL GIVE BRAINLIEST

Answers
b)For neutron,the formula is given by A=Z+N where A is the mass no=32 and Z is atomic no=16 and N is the neutron no=? So to find N , A-Z=N so 32-16 so neutron is 16
c)sulphur
in the combustion of methane, ch4(g)+ 2 O2(g) -> co2(g) +2 h2o (g), which reactant has the greatest rate of disappearance?
a. CO2
b. H2O
c. O2
d. CH4
e. CH4 and O2 have the same rate of disappearance
Answers
In the combustion of methane, CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g), the reactant that has the greatest rate of disappearance is O₂. The correct option is (c) O₂.
Combustion reactions are exothermic, in which the fuel, such as methane (CH₄), reacts with an oxidant, such as oxygen (O₂), to produce heat and other products, including carbon dioxide (CO₂) and water (H₂O).
This equation is balanced, meaning the number of atoms of each element is the same on both sides of the equation. In the above equation, methane and oxygen are the reactants, while carbon dioxide and water are the products. As a result, in the combustion of methane, oxygen is the reactant that disappears at the highest rate during the reaction.
Therefore, the correct answer is option (c) O₂.
Learn more about combustion on:
https://brainly.com/question/10458605
#SPJ11
What is chemical composition of ores, minerals, rocks, lime and mortar?
Answers
Minerals: The composition of a mineral can be expressed as a CHEMICAL FORMULA, which simply gives the proportions of the different elements and groups of elements in the mineral. The latter notion (groups of elements) comes into play for those minerals which have a restricted range of composition.
Rocks: Most rocks contain silicate minerals, compounds that include silica tetrahedra in their crystal lattice, and account for about one-third of all known mineral species and about 95% of the earth's crust. The proportion of silica in rocks and minerals is a major factor in determining their names and properties.
Lime: is a calcium-containing inorganic material composed primarily of oxides and hydroxide, usually calcium oxide and/or calcium hydroxide. It is also the name for calcium oxide which occurs as a product of coal-seam fires and in altered limestone xenoliths in volcanic ejecta.
Ores: Ore is natural rock or sediment that contains one or more valuable minerals, typically containing metals, that can be mined, treated and sold at a profit. Ore is extracted from the earth through mining and treated or refined, often via smelting, to extract the valuable metals or minerals.
Mortars: made from magnesia-phosphate cement were observed to set within 15 minutes at 22°C and to harden within 1 hour. The major hydrate formed was struvite, NH4MgPO4·6H2O, usually accompanied by schertelite, (NH42Mg(HPO4)2·4H2O, at least initially. Some hydration products also contained dittmarite, NH4MgPO4·H2O, and/or stercorite, NaNH4HPO4·4H2O, but these were present only as minor constituents.
Learn more about it from:
https://brainly.com/question/26072376
https://brainly.com/question/16681794?referrer=searchResults
Please I need this answer

Answers
Answer:
wave c i think
Explanation:
One lawn chair is made of aluminum (c=0.89 j/g°c) and another is made of iron (c=0.45 j/g°c). both chairs are painted the same color. on a sunny day, which chair you want to sit on? why?
Answers
The preferred chair to sit on a sunny day is the one made of aluminum. It offers a more comfortable seating experience compared to the iron chair. The aluminum chair's higher specific heat capacity helps it absorb less heat and stay cooler.
Why is the aluminum chair preferred on a sunny day?Aluminum is the preferred choice for sitting on a sunny day due to its higher specific heat capacity (c=0.89 J/g°C) compared to iron (c=0.45 J/g°C). Specific heat capacity refers to the amount of heat energy required to raise the temperature of a substance by one degree Celsius per gram.
When exposed to the sun, both chairs will absorb heat energy from the sunlight. However, aluminum has a higher specific heat capacity, meaning it can absorb more heat energy per gram compared to iron. This results in the aluminum chair heating up at a slower rate than the iron chair.
The slower rate of heat absorption by the aluminum chair makes it more comfortable to sit on during a sunny day. It will take longer for the aluminum chair to reach an uncomfortable temperature compared to the iron chair, providing a more pleasant seating experience.
Learn more about Aluminum Chair
brainly.com/question/29446016
#SPJ11
what forces act on a stationary bike
Answers
Answer:potential energy
Explanation:
because it is stationary
3. Convert 1976 kilograms to pounds
Answers
Answer:
4356.334 Pounds
Answer:
4356.334 pounds
Explanation:
I hope this is correct
Practice 55
Which hydrate contains the greatest
percent of water by mass?
1) LiCl H₂O
2) CaCl₂ 2H₂O
3) CuSO4 5H₂O
4) FeBr3 6H₂O
Answers
CaCl₂ 2H₂O contains the greatest percent of water by mass By halving the hydrate's formula , one may compute the theoretical (real) percent hydration.
What does it mean to hydrate?To provide with enough liquid or moisture; to make to occupy or mix with water or indeed the constituents of water. softens and moisturizes the skin. the intransitive verb "to hydrate"
What is a better hydrator than water?Milk is one of finest liquids for hydration, according to research, even more than water or caloric beverages. Researchers attribute milk's efficiency to its protein, carbs, and natural electrolytes.
To know more about hydrate visit :-
brainly.com/question/11202174
#SPJ1
When is the mole ratio from a balanced chemical reaction not needed in a calculation
Answers
Answer:16
Explanation:
Rank the following aqueous solutions from lowest predicted boiling point to highest: In the case of solutions containing aqueous ions, assume there is no ion clustering in the solution: 167 mCH_OH0.530 mNazSO4 0.475 mKaPO40.907 mKBr0.722 mNHANO3
Answers
From highest to lowest, the following aqueous solutions are: 0.475 m K3PO4 (highest), 0.907 m KBR, 1.67 m CH3OH, 0.530 m NA2SO4, and 0.722 m NH4NO3 (lowest).
The boiling point of a salt solution rises as the boiling point does. A Rhizob provides the majority of eubacterial antibiotics. As can be seen from the concentration figures, methanol has the lowest concentration. Methanol will have the lowest boiling point as a result. The solution of 0.10 M SrCl2 in 300.0 mL contains the most ions as a result. Therefore, 1.0 MNa2SO4 has the maximum boiling point value. The most highly vaporized substance is 1-chloropentane. Because of its boiling point's "propto 1/("branching")," tert-butyl alcohol has the lowest boiling point. Surface area decreases with increasing branching.
Learn more about aqueous solutions here:
https://brainly.com/question/26856926
#SPJ4
Of the following, which are true of nuclei that are to the left or right of the band of stability? Select all that apply: They are known as radioisotopes. They do not undergo nuclear decay. They are considered stable. They undergo nuclear decay.
Answers
Required true statements are They are known as radioisotopes and hey undergo nuclear decay.
Of the following statements, the true ones about nuclei that are to the left or right of the band of stability are:
1. They are known as radioisotopes.
4. They undergo nuclear decay.
Nuclei that are outside the band of stability are considered unstable and are called radioisotopes. They undergo nuclear decay to reach a more stable state. The other two statements (they do not undergo nuclear decay and they are considered stable) are false for nuclei outside the band of stability.
Learn more about radioisotopes here,
https://brainly.com/question/18640165
#SPJ11
The photograph below shows the East African Rift Valley in Africa. Which tectonic movement
of Earth’s crust is most likely responsible for this feature?
Answers
Answer:
divergence of continental crust
Explanation:
7. Cigarette smoke and UV radiation are two
examples of a
Answers
Answer:
carcinogen
Explanation:
cigarette smoke and UV radiation both of the capability to cause cancer cells in living tissue, meaning they can both be classed as carcinogens
3. When 2. 750 g of Pb3O4 is heated to a high temperature, it decomposes to produce 0. 0640 g of
oxygen gas and 2. 686 g of some new lead oxide compound. Use this data to determine the
empirical formula of the new lead oxide compou
Answers
The empirical formula of the new lead oxide compound is PbO₇.
We can use the given data to determine the moles of Pb₃O₄, oxygen gas, and the new lead oxide compound produced,
moles of O₂ = 0.0640 g / 32.00 g/mol = 0.00200 mol
moles of new lead oxide compound = (2.686 g - 2.750 g) / 223.2 g/mol = -0.000287 mol
Now we can use the moles of oxygen gas and the new lead oxide compound to determine the empirical formula of the new lead oxide compound. From the balanced chemical equation for the decomposition of Pb₃O₄,
Pb₃O₄ → 3PbO + 0.5O₂
we see that 0.5 mol of O₂ is produced for every 3 mol of PbO produced. Therefore, the mole ratio of O₂ to the new lead oxide compound is,
0.00200 mol O₂ / -0.000287 mol new lead oxide compound ≈ -6.96
To get integer mole ratios, we can multiply both sides of the ratio by a common factor to obtain whole numbers. In this case, multiplying by 7 gives.
0.0140 mol O₂ / -0.00201 mol new lead oxide compound ≈ -7
This means that for every 7 moles of oxygen gas produced, there are approximately 1 mole of the new lead oxide compound produced.
The empirical formula of the new lead oxide compound can be expressed as PbOₓ. Since we know that the ratio of Pb to O in the compound is 1 : x, and we have determined that the ratio of O to the new lead oxide compound is approximately 7:1,
PbO₇ ≈ PbOₓ
To know more about empirical formula, here
brainly.com/question/14044066
#SPJ4
--The complete question is, When 2.750 g of Pb3O4 is heated to a high temperature, it decomposes to produce 0. 0640 g of oxygen gas and 2. 686 g of some new lead oxide compound. Use this data to determine the empirical formula of the new lead oxide compound.--
1 point
A system that absorbs energy from its surroundings is said to be: *

Answers
Answer: Exothermic.
Explanation:
Exo means “outside” and the outside relates to surroundings so it’s my best choice.
Answer:
endothermic is the answer
Sara wants to see if a new brand of hair dye lasts longer than the brand she currently uses. She puts the new hair dye on the left side of her head and the old brand on her right side. After 2 weeks she observes which side of her head has more gray hair showing through.
a. Independent Variable =
b. Dependent Variable =
c. Constant =
Answers
Answer:
a: the hair dye
b: the change in color of her hair
c: the same amount of hair for both dyes and the same amount of dye
Explanation:
Boron trifluoride (BF3) is a polar molecule containing polar bonds. True/False?
Answers
True.
Boron trifluoride (BF3) is a polar molecule because it contains polar covalent bonds and the molecular geometry of the molecule is trigonal planar.
The boron atom has a partial positive charge while the fluorine atoms have a partial negative charge due to the electronegativity difference between them. This creates a net dipole moment in the molecule making it polar.
To know more about types of bonds, click here:-
https://brainly.com/question/10777799
#SPJ11
give 10 examples of potential energy converted into kinetic energy
Answers
Answer:
Book on Table
Car at the Hilltop
Falling Objects
Skydiver
Hammering a Nail
Dam Water
Roller Coaster
Stretched Rubber Band
Simple Pendulum
Compressed Spring
Battery
Flashlight
Exothermic Chemical Reaction
Burning of Oil, Gas and Coal
Wind Turbine
Explanation:
have a nice day!
Write a simple procedure for measuring out 2.6 grams of sodium bicarbonate NaHCO3 or commonly known as baking soda?
PLEASE I REALLY NEED THIS!!! :/
Answers
Answer:
See explanation
Explanation:
If I intend to measure 2.6 g of sodium bicarbonate, I need a spatula, a filter paper, and a weighing balance.
The filter paper is placed on the balance and the balance is adjusted to read zero. The spatula is now used to collect the sodium bicarbonate solid from its container and gradually drop it on the filter paper while watching the mass read out by the balance. This continues until the balance reads 2.6g.
What is the formula for sulfate?
What is the charge of sulfate?
What is the correct formula for copper (II) sulfate?

Answers
Answer:
SO₄²-, -2, CuSO4
Explanation:
Dang that was long.
What is the general form of a synthesis reaction?
O A. AB + CD → AC + BD
B. A + B → AB
O C. AB + C → AC + B
D. AB → A+B
Be
The answer is B
Answers
Answer:
B. A+ B → AB
Explanation:
general form of a synthesis reaction
Which of the following equations follows the Law of Conservation of Mass? 65
(5 Points)
3H2O --> 3H2 + 3 02
C2H4 + 3 02 --> 2 CO2 + 2 H2O
C + 4H2 --> CH4
2Na + Cl --> NaCl
ILL MARK YOU AS TOP COMMENT
Answers
Answer:
C₂H₄ + 3O₂ → 2CO₂ + 2H₂O
Explanation:
According to the law of conservation mass, mass can neither be created nor destroyed in a chemical equation.
This law was given by French chemist Antoine Lavoisier in 1789. According to this law mass of reactant and mass of product must be equal, because masses are not created or destroyed in a chemical reaction.
3H₂O → 3H₂ + 3O₂
This equation do not follow the law of conservation of mass because there are more oxygen atoms on right side so mass is not conserved.
C₂H₄ + 3O₂ → 2CO₂ + 2H₂O
This equation follow the law of conservation of mass because there are equal number of atoms of all elements on both side of equation.
C + 4H₂ → CH₄
This equation do not follow the law of conservation of mass because there are more hydrogen atoms on left side of equation so mass is not conserved.
2Na + Cl → NaCl
This equation also do not follow the law of conservation of mass because there are more sodium atoms on left side of equation so mass is not conserved.
how many atoms are there in 2 moles of oxygen molecules?
Answers
There are 12.044 × 10^23 oxygen molecule present in 2mole of oxygen molecule.
According to the context, the word may as well as may not encompass ions that meet this requirement. A molecule is a collection of two as well as more atoms linked together by the attractive forces described as chemical bonds.
The lowest unit into which a substance could be divided while still being the same substance would be a molecule. It is composed of two as well as more atoms which are chemically bonded to one another.
1 mole of oxygen = 1 O2 molecule
2 mole = 2 O2 molecule = 2 × 6.022 × 10^23 molecule = 12.044 × 10^23 molecule
Thus, 2 mole of oxygen will have 12.044 × 10^23 molecule
Tp know more about molecule
https://brainly.com/question/19922822
#SPJ4
what is a reaction that uses a catalyst?
Answers
One of the most commonly known catalysed reaction is that of Hydrogen Peroxide turning into Water
In this reaction, we can use Potassium Permanganate (KMnO₄) or Palladium (Pd) as a catalyst.
These catalysts helps Hydrogen Peroxide turn into water and oxygen
Catalyst: Substances that change the rate of a chemical reaction and themselves remain chemically unchanged after the reaction are called catalysts.
Example:- In the process of manufacturing of ammonia Fe is used as Catalyst.
\(N _{2} (g)+ 3H _{2} (g) \huge \rightarrow {}^{Fe}\rightarrow \small{3NH _{3}(g)}\)
Note:- there is only one arrow. I was unable to put iron over the arrow ..
Hope This Helps You ❤️Using your periodic table which Adam below has the most similar characteristics to the atom potassium the atomic number of 15?
A Sulfur
B Nitrogen
C silicone
D carbon
Answers
Answer:
B. Nitrogen
Explanation:
Nitrogen is in the same group as potassium, meaning they have the same number of valence electrons. The atoms with the same number of valence electrons have similar chemical characteristics; therefore, potassium and nitrogen are most similar.
what is the conjugate base of a weak acid?group of answer choicesnegligible baseweak basestrong basespectator ionweak acidstrong acidnegligible acid
Answers
The conjugate base of a weak acid is a weak base and the conjugate base of a strong acid is a strong base.
The conjugate base of a weak acid is the species that is formed when the acid donates a proton (H+) to another species. It is the species that remains when the acid has lost its proton.
A weak acid is one that has a low dissociation constant and so, weakly donates its proton to a base. This means that the conjugate base of a weak acid will also be a weak base.
Examples of weak acids include acetic acid (CH3COOH), citric acid (C6H8O7), and carbonic acid (H2CO3).
The conjugate base of a weak acid is the species formed when a proton is accepted by a base.
The base used to accept the proton can be either a weak or a strong base. When a weak base accepts a proton from a weak acid, the result is a weak conjugate base.
Examples of weak bases include ammonia (NH3), hydroxide ion (OH-), and carbonate ion (CO3-2).
The conjugate base of a strong acid is a strong base.
This is because a strong acid completely dissociates into its ions in aqueous solution and the conjugate base of the acid is the species that remains.
Examples of strong acids include hydrochloric acid (HCl), nitric acid (HNO3), and sulfuric acid (H2SO4).
Examples of strong bases include sodium hydroxide (NaOH), potassium hydroxide (KOH), and calcium hydroxide (Ca(OH)2).
In summary, the conjugate base of a weak acid is a weak base and the conjugate base of a strong acid is a strong base.
To know more about weak bases, refer here:
https://brainly.com/question/29833185#
#SPJ11
help me with this ?
i need to git this done for school
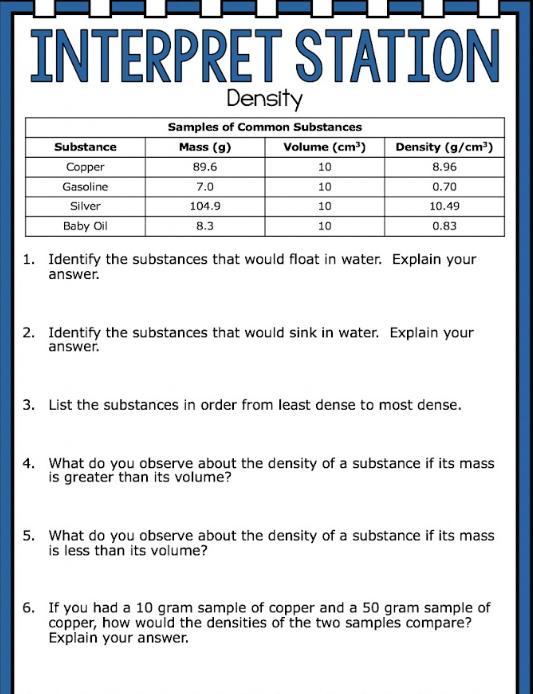
Answers
1) Gasoline and baby oil will float in water
2) Copper and silver will sink in water
3) Silver, Copper, Baby oil Gasoline
4) If the mass is less than volume the density will be less than one
5) If the mass is greater than the volume the density is not less than 1
6) The densities of the substances would be the same.
What is the density?Density is a physical property of matter that measures how much mass is contained in a given volume. It is calculated as the ratio of mass to volume, and is typically expressed in units of grams per cubic centimeter (g/cm³) or kilograms per cubic meter (kg/m³).
The formula for calculating density is:
Density = Mass / Volume
Where Mass is the amount of matter in an object or substance, and Volume is the amount of space that it occupies. Density is an intensive property, which means that it is independent of the amount of substance being measured. For example, the density of a material will be the same regardless of whether you measure it in grams or kilograms.
Learn more about density:https://brainly.com/question/29775886
#SPJ1
what will be the result of the reaction
(CH3COO)2+redP +Cl2
Answers
Answer:
(CH3COO)2 + redP + Cl2 → ClCH2COOH + HCl
Explanation:
This is an example of halogenation of carboxylic acids at alpha carbon atom. In this reaction, red phosphorus and chlorine are treated with carboxylic acids having alpha hydrogen atom followed by hydrolysis to form alpha chloro carboxylic acid.
help me name the molecule

Answers
Answer:
ether
Explanation:
ether is -OR
- Hope that helps! Please let me know if you need further explanation.