what mass of ZnCI2 can be prepared from the reaction of 3.27 grams of zinc with 3.30 grams of HCI?
Zn + 2HCI —-> ZnCI2 + H2
Answers
Answer:
Zn. + 2 HCl ----------> ZnCl2. + H2
Explanation:
Mass of Zn in mole = 3.27/ 65.38= 0.050010.... mol
Mass of HCl in mole = 3.3/ 73= 0.044....mol
Hence, limiting reagent is HCl
Molar mass of ZnCl2= 136.38g
Let mass of ZnCl2 be x
x = 3.3*136.38÷ 73 = 6.165...
So, the mass of ZnCl2 is 6.17 g
Related Questions
consider the molecules scl2, f2, cs2, cf4, and brcl. (a) which has bonds that are the most polar? [ select ] (b) which of the molecules have dipole moments?
Answers
Answer:
CF₄ has the most polar bonds among the molecules and SCl₂ and BrCl have dipole moments.
Explanation:
(a)To determine which molecule has the most polar bonds, we need to consider the electronegativity difference between the atoms in each molecule. The greater the electronegativity difference, the more polar the bond. Here are the electronegativity values for the elements involved:
Sulfur (S): 2.58
Fluorine (F): 3.98
Carbon (C): 2.55
Bromine (Br): 2.96
Chlorine (Cl): 3.16
Comparing the electronegativity differences:
SCl₂: The electronegativity difference between sulfur (2.58) and chlorine (3.16) is 0.58.
F₂: The electronegativity difference between fluorine (3.98) and fluorine (3.98) is 0.
CS₂: The electronegativity difference between carbon (2.55) and sulfur (2.58) is 0.03.
CF₄: The electronegativity difference between carbon (2.55) and fluorine (3.98) is 1.43.
BrCl: The electronegativity difference between bromine (2.96) and chlorine (3.16) is 0.2.
From the calculations, we can see that CF₄ has the highest electronegativity difference (1.43) and, therefore, the most polar bonds.
(b) To determine which of the molecules have dipole moments, we need to consider the molecular geometry and the polarity of the bonds.
SCl₂: Sulfur dichloride (SCl₂) has a bent molecular geometry. The S-Cl bonds are polar, and due to the bent shape, the dipole moments do not cancel out, resulting in a net dipole moment.
F₂: Fluorine (F₂) is a diatomic molecule with a linear shape. The bond is nonpolar because the electronegativity of fluorine is the same, and the dipole moments cancel each other out.
CS₂: Carbon disulfide (CS₂) has a linear molecular geometry. Although the C-S bonds are polar, the molecule is symmetrical, and the dipole moments cancel out, resulting in a nonpolar molecule.
CF₄: Carbon tetrafluoride (CF₄) has a tetrahedral molecular geometry. The C-F bonds are polar, but the molecule is symmetrical, and the dipole moments cancel out, resulting in a nonpolar molecule.
BrCl: Bromine chloride (BrCl) has a linear molecular geometry. The Br-Cl bond is polar, and the molecule does not possess symmetry to cancel the dipole moments. Hence, it has a net dipole moment.
Learn more about dipole moment here, https://brainly.com/question/11626115
#SPJ11
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed. (i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution. Initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction
Answers
Answer:
it is an endothermic reaction
Explanation:
This is because there is a rise in temperature from 20 to 46. this means that the reaction takes in heat from the suuroundings
Students measuring the mass of a metal block record 2.985 g, 3.051 g, 3.102
g, and 3.211 g. Which other measurement of the block's mass would be most
precise?
A. 2.804 g
B. 3.1159
C. 3.418 g
D. 3.509 g
Answers
Answer:C - 3.115 g
Explanation:Correct
2) A student determined the melting point of a substance to be 55.2DC. If the accepted value
is 50.1C, what is the percent error in the student's determination?
A) 12.0 B) 5.10 C) 10.2 D) 9.24
Answers
Answer:
C) 10.2
Explanation:
Formula: Actual-Expected/Expected
The actual was 55.2 while the expected was 50.1
55.2-50.1/50.1=0.101796407 * 100= 10.2%
The percentage error in the student's determination is 10.2%
The correct answer to the question is Option C. 10.2
From the question given above, the following data were obtained:
Measured value = 55.2 °C
Accepted value = 50.1 °C
Percentage error =?Next, we shall determine the absolute error. This can be obtained as follow:
Measured value = 55.2 °C
Accepted value = 50.1 °C
Absolute error =?Absolute error = |Measured – Accepted|
Absolute error = |55.2 – 50.1|
Absolute error = 5.1Finally, we shall determine the percentage error.
Absolute error = 5.1 °C
Accepted value = 50.1 °C
Percentage error =?\(Percentage error = \frac{Absolute error }{Accepted value} * 100\\\\= \frac{5.1}{50.1} * 100\\\\\)
Percentage error = 10.2%Therefore, the percentage error in the student's determination is 10.2%.
The correct answer to the question is Option C. 10.2
Learn more: https://brainly.com/question/17441904
Here’s the second part to my science

Answers
Answer:
"powerhouse"- mitochondria
"stores spare parts"- vacuole
"surrounds the nucleus"- nuclear membrane
"controls the cell"- nucleus
"pathways"- endoplasmic reticulum
"inner layer"- cell membrane
"outer plant covering"- cell wall
"fills up the cell"- ctyoplasm
"collects light"- chloroplasts
"packages proteins"- golgi
"holds info"-chromosomes
"makes protein"-ribosomes
please mark me brainliest if you like my answer <3
2C4H10 + 13O2 Right arrow. 8CO2 + 10H2O
What is the mole ratio of butane to carbon dioxide?
Answers
2C4H10 + 13O2 Right arrow. 8CO2 + 10H2O
What is the mole ratio of butane to carbon dioxide?
ANSWER:
Butane is an organic compound with the formula C4H10 and carbon dioxide is compound (gas) with the formula CO2. So, the balanced coefficient of butane is 2, and that of carbon dioxide is 8. According to thid, the mole ratio of butane to carbon dioxide is 2:8 or 1:4.
Answer:
answer is 2:8 or 1:4
Explanation:
In what organelle of the cell does β oxidation of fatty acids take place?
chloroplasts
cytosol
nucleus
mitochondria
Golgi apparatus
Answers
β oxidation of fatty acids takes place in the mitochondria, which are organelles found in eukaryotic cells responsible for energy production. The process involves breaking down fatty acids into acetyl-CoA molecules, which can then be used by the cell to produce ATP through the process of cellular respiration.
The β oxidation pathway is important for providing energy to cells, especially during times of low glucose availability. Other organelles, such as the chloroplasts and the Golgi apparatus, have different functions and are not involved in this process. The cytosol, or the fluid portion of the cytoplasm, is the site of many metabolic processes but does not play a direct role in β oxidation. The nucleus is responsible for storing and protecting genetic material and is not involved in energy production.
The β-oxidation of fatty acids takes place in the mitochondria, an organelle within the cell. This process involves breaking down fatty acids into smaller molecules called acetyl-CoA, which are then used to generate energy through the citric acid cycle and oxidative phosphorylation. Mitochondria are often referred to as the "powerhouses" of the cell due to their role in energy production.
For more information on β oxidation visit:
brainly.com/question/31447382
#SPJ11
What is the process of separating liquids by boiling points?
Answers
Answer:
Fractional distillation (often shortened in high school to just distillation)
Explanation:
Some elements have different boiling points and can be separated by boiling one element out and condensing it. This is how sodium acetate is made, for example.
Hope this helped!
Please help
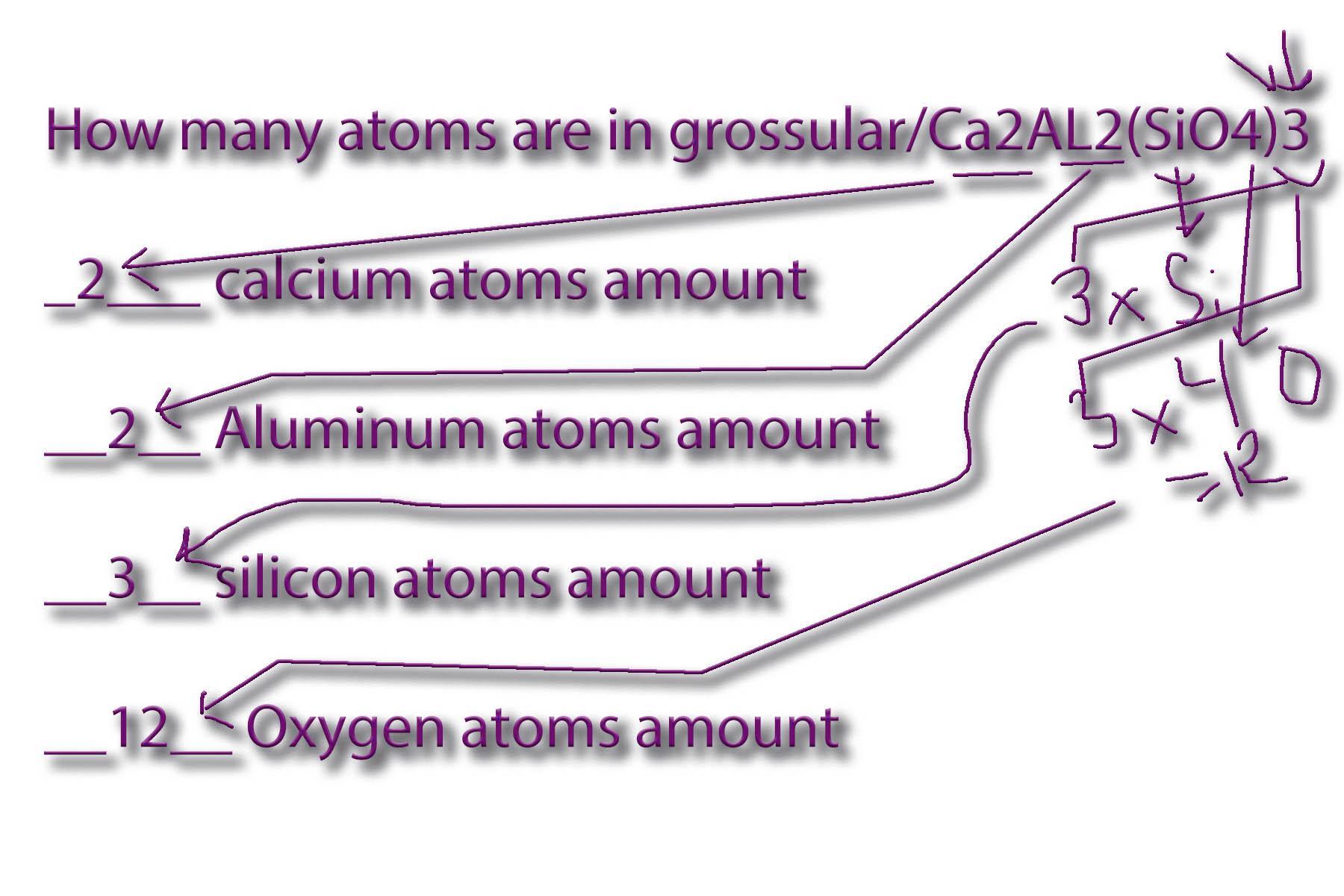
How many atoms are in grossular/Ca2AL2(SiO4)3
____ calcium atoms amount
____ Aluminum atoms amount
____ silicon atoms amount
____ Oxygen atoms amount
Answers
Answer:
See below
Explanation:
How many atoms are in grossular/Ca2AL2(SiO4)3
_2___ calcium atoms amount
__2__ Aluminum atoms amount
__3__ silicon atoms amount
__12__ Oxygen atoms amount
See attached worksheet
What is the pH of a solution with a [H3O+] concentration of 3.4 x 10-¹¹ M?
Answers
The pH of the solution will be 10.47.
what is pH?The pH of a solution is mathematically given as:
pH = - log [\(H^+\)] of -log [\(H_3O^+\)]
Thus, in this case, with [\(H_3O^+\)] of 3.4 x 10-¹¹ M:
pH = -log 3.4 x \(10^-^1^1\) = 10.47
Thus, the pH of the solution will be 10.47.
More on pH can be found here: https://brainly.com/question/15289741
#SPJ1
In the Millikan oil droplet experiment, the oil is sprayed from an atomizer into a chamber. The droplets are allowed to pass through the hole into the chamber so that their fall can be observed. The top and bottom of the chamber consist of electrically charged plates. The upper plate is positively charged, and the lower plate is negatively charged. X rays are introduced into the chamber so that when they strike the oil droplets, the droplets will acquire one or more negative charges. The electric field (voltage) is applied to the metal plates.
Watch the animation and identify the effects of an electric field on the motion of a negatively charged oil droplet. Consider the gravitational force as Fg and the electric force as Fe. All the other forces acting on the oil droplet can be ignored as their effect on the motion of the oil droplet is negligible.
A/ In the absence of an electric field, the oil droplet falls freely due to the gravitational force.
B/ If Fe is increased until it is equal to Fg, the negatively charged oil droplet will remain stationary.
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
D/ In the presence of an electric field, the negatively charged oil droplet moves freely toward the negatively charged plate.
** I chose B, but that was the wrong answer
Answers
C/ If Fe is greater than Fg, the negatively charged oil droplet will move freely toward the negatively charged plate.
In the Millikan oil droplet experiment, the negatively charged oil droplets are subjected to an electric field created by the charged plates. The electric force (Fe) acts on the oil droplet in a direction opposite to the gravitational force (Fg). When Fe is greater than Fg, the electric force overcomes the gravitational force, causing the negatively charged oil droplet to experience an upward force. As a result, the oil droplet moves freely upward toward the negatively charged plate.
Option B is incorrect because if Fe is equal to Fg, the forces balance each other, resulting in a stationary droplet. However, the question states that Fe is increased until it is greater than Fg, implying that the droplet is no longer stationary but moves in response to the electric force.
Therefore, option C is the correct answer, as it describes the effect of an electric field on the motion of a negatively charged oil droplet in the Millikan oil droplet experiment.
To learn more about Millikan oil droplet experiment, here
https://brainly.com/question/32330429
#SPJ4
Write the balanced equation for the reaction that occurs when methanol, ch3oh (l), is burned in air. What is the coefficient of methanol in the balanced equation?.
Answers
The balanced equation is given as :
2CH₃OH + 3O₂ ----> 2CO₂ + 4H₂O , The coefficient of methanol is 2.
The reaction of the methanol that is CH₃OH , when it is burned in the presence of the air is given below :
CH₃OH + O₂ ----> CO₂ + H₂O
To balanced equation , the number of the atoms in the reactant side is equals to the product side.
Reactant Product
C 1 1
H 4 2
O 3 3
to balanced the equation , multiply the , 2 in CH₃OH , 3 in O₂ and the 2 in CO₂ and the 4 in H₂O. we get :
2CH₃OH + 3O₂ ----> 2CO₂ + 4H₂O
The above equation is now balanced. The coefficient if methanol is 2.
To learn more about balanced equation here
https://brainly.com/question/4531968
#SPJ4
I need help assapppp





Answers
Answer:
Lithium has 3 protons, 4 neutrons, 3 electrons, and its mass is 6.941 u
can someone explain how to do this, I don't understand?

Answers
Answer:
a) 2
b) 3
c) 5
d) 6
Explanation:
is soulubility maseruble physical property
Answers
When performing an extraction with between an aqueous solution and organic solution what determines which layer ends up the bottom layer in the separatory funnel ?.
Answers
When performing an extraction between an aqueous solution and an organic solution or organic compound, density will determine the layer ending up at the bottom of the separation funnel.
In chemistry, a separatory funnel is a kind of funnel that separates two immiscible liquids.
It is a transparent funnel and when two immiscible liquids i.e aqueous and organic solution is poured into it, and then allowed to stand, after a while a distinguished layer is formed setting them apart from each other.
Due to the difference in density, the extraction becomes easier as the aqueous layer has a low density and stays at the top while the organic layer is at the bottom.
If you need to learn more about organic compounds click here:
https://brainly.com/question/19083306
#SPJ4
Which of the following is the energy of motion? O Elastic energy O Gravitational energy O Kinetic energy O Potential energy
Answers
Answer:
kinetic energy
Explanation:
all moving objects have kinetic energy. when an object is an motion it changes its position by moving in a direction: up,down, forward, or backward
Answer: its C kinetic energy
Explanation: i did the test
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons.
Answers
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. Therefore, the given statement is correct is true.
What is electron sea model?The Electron Sea Model's whole hypothesis relies around the behavior of atoms throughout this bonding. The movement of unpaired electrons between positively charged metal ions in a mesh is known as metallic bonding.
According to the electron sea model, the melting points of metals are not as extreme as the boiling points. This is because the cations and electrons are - in a metal. It does not take much energy for a solid to become liquid. But metallic bonds are very - , so it does require a lot of energy to separate atoms from the cations in their sea of electrons. This statement is true.
Therefore, the given statement is correct is true.
To know more about electron sea model, here:
https://brainly.com/question/30003484
#SPJ1
During ionic bonding, there must be a chemical interaction between...
O metallic elements, only
O nonmetallic elements, only
O one metallic and one nonmetallic element, only
O metalloids ,only
Answers
plz help fast! P.S. I put chemistry by accident its biology lol
The measure of water’s ability of flow through sediment and rock is called
Answers
Answer:
Permeability.
Explanation:
The ability of a rock or soil to allow water to flow through it is call permeability. Materials such as gravel that allow the flow of water are permeable. Materials such as clay or granite that do not allow the flow of water are impermeable.
Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
For the following situations, describe the contact and non contact forces that are involved.
A. A book sitting on a shelf
B. A soccer player kicking a ball; the ball soaring through the air and landing on the ground
C. A hiker in the woods reading her compass to determine which direction to go
D. A child on a sled; first, the child is sitting at the top of the hill, waiting his turn; then, the child pushes off and slides down the hill, eventually coming to a ston
Answers
Answer:
D
Explanation:
Bc I got it right
Answer:
For A: the contact I think would be the bookshelf holding it up the non-contact force would be gravity making it so the book would stay where it was put.
For B: the contact force would be the ball being kicked and the non-contact would be the gravity pulling it back to the ground.
For C: A hiker in the woods reading her compass to determine which direction to go The contact would be her holding the compass and moving around the non-contact would be the compass arrow turning every time she moves.
For D: The child on a sled for the contact force would be the person pushing the little kid down the hill the non-contact force would be the gravity that would be keeping the kid going down the hill and gravity stopping the kid at the bottom of the hill.
Explanation:
Have the same assignment and these are the answers I gave. Hope this helps someone!
For every 6 mols of H2 how many mols of h20 will be produced?
For every 2 mols of H2 how many mols of h20 will be produced?
For every 5.67 mols of H2 how many mols of h20 will be produced?
Answers
The moles would be 12 moles, 4 moles and 11.34 moles
How to solve for the molesWhen hydrogen gas (H2) reacts with oxygen gas (O2) to produce water (H2O), the balanced chemical equation is:
2H2 + O2 -> 2H2O
So,
For every 6 moles of H2, 12 moles of H2O will be produced
For every 2 moles of H2, 4 moles of H2O will be produced
For every 5.67 moles of H2, 11.34 moles of H2O will be produced.
Read more on the moles here:https://brainly.com/question/15356425
#SPJ1
Calcium sulfate, CaSO4, has a Ksp value of 7.10×10−5 . What happens when calcium and sulfate solutions are mixed to give 2.00×10−3M Ca2+ and 3.00×10−2M SO42−?
Calcium sulfate, , has a value of 7.10×10−5 . What happens when calcium and sulfate solutions are mixed to give 2.00×10−3 and 3.00×10−2 ?
A.) A precipitate forms because Q>Ksp.
B.) A precipitate forms because Q
C.) No precipitate forms because Q>Ksp.
D.) No precipitate forms because Q
Answers
No precipitate forms because Q < Ksp. Therefore, option (D) is correct.
To determine what happens when calcium and sulfate solutions are mixed, we need to compare the reaction quotient (Q) with the solubility product constant (Ksp) for calcium sulfate (CaSO₄).
The balanced equation for the dissolution of calcium sulfate is:
CaSO₄(s) ⇌ Ca²⁺(aq) + SO₄²⁻(aq)
The expression for Q is given by:
Q = [Ca²⁺][SO₄²⁻]
Given that the concentrations of Ca²⁺ and SO₄²⁻ are 2.00×10⁻³ M and 3.00×10⁻² M, respectively, we can substitute these values into the Q expression:
Q = (2.00×10⁻³)(3.00×10⁻²)
= 6.00×10⁻⁵
Comparing Q with the Ksp value of calcium sulfate (7.10×10⁻⁵), we find that Q is smaller than Ksp (Q < Ksp).
In this case, when Q is smaller than Ksp, it indicates that the ionic product of the concentrations of Ca²⁺ and SO₄²⁻ is less than the solubility product. Therefore, no precipitate will form because the solution is not yet saturated with respect to calcium sulfate.
Thus, the correct option is (D).
Learn more about Ksp, here:
https://brainly.com/question/23719355
#SPJ12
A sample of gas occupies 2.30 L at 825 mmHg and 70.0°C. What is its volume at STP
Answers
Answer:
V₂ = 2.0 Liters at STP conditions
Explanation:
Solving problem using the combined gas law
Given:
Case I Conditions Cast II Conditions
P₁ = 825mmHg P₂ = 760mm
V₁ = 2.30 Liters V₂ = ?
T₁ = 70°C + 273 = 343K T₂ = 0°C = 273K
Substitute into combined gas law assuming moles of gas remains constant; solve for unknown volume under case 2 conditions
P₁·V₁/n₁·T₁ = P₂·V₂/n₂T₂ => V₂ = P₁·V₁·T₂ / P₂·T₁ => note: in this solution, moles of gas remains constant as is disregarded in final calculation.
V₂ = (825mm)(2.3L)(273K) / (760mm)(343K) = 1.987 Liters (calculator answer) ≅ 1.9 Liters (2 sig figs based on given volume (V₁)
V₂ = 1.987 Liters ≅ 2.0 Liters (2 sig figs based on given volume (V₁)
A reaction vessel contains 20.0 grams of sodium metal and 10.0 grams of chlorine gas. The reaction to produce sodium chloride has gone to 100% completion. Which is the excess reagent and how much of it remains
Answers
Sodium metal is the excess reagent and after the completion of the reaction 16.8g of it is left.
What is excess reagent?In a chemical reaction, the reactant which is present in excess and don't get fully used up in the reaction is called excess reagent.
How to calculate excess reagent?To calculate excess reagent we will use the formula:no. of moles (n)/stoichiometry coefficient.
The reactant with greater value will be the excess reagent.
The balanced chemical equation for the reaction is:\(2Na+Cl_2\) ⇒ \(2NaCl\)
where given mass of Na is 20g molecular weight=23g
given mass of \(Cl_{2}\) is 10g molecular weight=71g
{n/S.C} for Na: {n/S.C} for Cl:n=given mass/ molar mass n=given mass/ molar mass
=20/23 =10/71
S.C=2 S.C.=1
so n/S.C value is 0.43 so n/S.C value is 0.14
Since value is high, Na is Since value is lower, \(Cl_{2}\) excessive reagent. is limiting reagent.
The completion of reaction will depend on the limiting reagent.according to the equation, 71g of \(Cl_{2}\) react with 23g of Na
1g ≡ 23/71
10g ≡ 23/71 *10
=3.2g of Na
the amount of Na left is 20-3.2 =16.8g
Hence Na is the excess reagent with 16.8g of it remains.
For more information refer to:
https://brainly.com/question/14222359
#SPJ4
When using IR spectroscopy, what is being recorded by the machine?
Sizes
O Wavelengths
O Color
O Molecular mass
Answers
Answer:
wavelengths
Explanation:
hope it helps
thanks
Wavelengths are being recorded by the machine by using IR spectrography. Therefore, option B is correct.
Infrared (IR) spectroscopy is also known as infrared spectrometry. It is a technique that is used to analyze and identify chemical compounds based on their absorption or emission of infrared radiation.
Infrared radiation lies in the electromagnetic spectrum between visible light and microwave radiation. It has longer wavelengths and lower frequencies than visible light. When infrared radiation passes through a sample, certain chemical bonds in the sample absorb specific frequencies of infrared light.
Learn more about spectrography, here:
https://brainly.com/question/30917323
#SPJ2
Properties and Uses of Unsaturated Hydrocarbons
Project: Communicating Design Details
Active student guide
Answers
Answer:
Welcome to the project on communicating design details for the properties and uses of unsaturated hydrocarbons. This project aims to enhance your understanding of the characteristics and applications of unsaturated hydrocarbons.
Here are the steps to complete this project:
Step 1: Research
Research the different types of unsaturated hydrocarbons, including alkenes and alkynes. Find out their general properties, such as their reactivity, flammability, and solubility. Also, identify their uses in various industries, such as plastics, rubber, and fuel.
Step 2: Create a Design
Using your research findings, create a design to visually communicate the properties and uses of unsaturated hydrocarbons. You can use tools like Canva, PowerPoint, or other design software to create infographics, posters, or slideshows.
Step 3: Incorporate Key Information
Incorporate the key information you gathered in step 1 into your design. Make sure to include the following details:
Definitions of unsaturated hydrocarbons, alkenes, and alkynes
Properties of unsaturated hydrocarbons, including reactivity, flammability, and solubility
Applications of unsaturated hydrocarbons in various industries, such as plastics, rubber, and fuel
Examples of unsaturated hydrocarbons, such as ethene and propene for alkenes, and ethyne for alkynes
Step 4: Review and Refine
Review your design and refine it to make sure it effectively communicates the properties and uses of unsaturated hydrocarbons. Check for spelling and grammar errors, and ensure that the information is accurate and easy to understand.
Step 5: Present Your Design
Present your design to your class or teacher, and explain the properties and uses of unsaturated hydrocarbons. You can also invite feedback and questions to enhance your understanding of the topic.
In conclusion, the properties and uses of unsaturated hydrocarbons are essential for many industries. Through this project, you will gain a better understanding of unsaturated hydrocarbons and develop your communication skills to effectively present your findings. Good luck!
Explanation:
Answer:
Explanation:
The three types of unsaturated hydrocarbons is alkynes, alkenes, and aromatic hydrocarbons. Which is composed of alkynes? acetylene. brainlist
Please help!!! This is due later today and I have no idea how to do this

Answers
Answer:
If you have a periodic table of the elements, you should be able to do this easily. The best periodic table, in my opinion, is the interactive periodic table at ptable(.com).
I will attach a formula sheet with everything you might need to know in chemistry.
Explanation:
You should be able to do this, but if you need more help let me know! :)
Number the steps in the correct order to show how ice changes into gas. __ The molecules gain enough kinetic energy to leave their solid form. __ The water becomes liquid and gets hotter. __ Ice is heated and the molecules speed up __ The molecules leave their liquid form and enter the air as a gas. __ Ice molecules vibrate in a fixed place.
Answers
Answer: Please find answer in explanation column
Explanation:
Numbering the steps in the correct order to show how ice changes into gas, we have that
1.Ice molecules vibrate in a fixed place.
2. Ice is heated and the molecules speed up
3. The molecules gain enough kinetic energy to leave their solid form.
4.The water becomes liquid and gets hotter.
5. The molecules leave their liquid form and enter the air as a gas.
Ice is the solid form of water which cannot freely move around in this form so it vibrates about its fixed position.When heated,its molecules acquire energy and its vibration speeds up causing it to overcome its binding forces (that makes it vibrate about a fixed position) and becomes mobile as it leaves its solid form to acquire a liquid state. With continuous heating, the liquid gets hotter making the molecules vibrate more rapidly, spread out and move freely at high speeds to form Water vapour - The gaseous state of water.