What part of the sun do we see from earth?
Answers
Answer:
photosphere
Explanation:
photosphere
There are 3 main layers of the Sun that we can see. They are the photosphere, the chromosphere and the corona. Together they make up the "atmosphere" of the Sun. The part of the Sun that glows (and that we see with the naked eye) is called the photosphere
Answer:
There are 3 main layers of the Sun that we can see. They are the photosphere, the chromosphere and the corona. Together they make up the "atmosphere" of the Sun. The part of the Sun that glows (and that we see with the naked eye) is called the photosphere.
Explanation:
Yes, that's right a layer of the sun is called Corona
Coincidence, I think not
Related Questions
A HgO → Hg + O2
B 2Fe + O2 → Fe2O3
Which best represents a
balanced equation?
C 2H2 + O2 → 2H20
Answers
Reaction Data
Reactants Products
Al(NO3)3 NaCl NaNO3 AlCl3
Starting Amount in Reaction 4 moles 9 moles ? ?
Determine the maximum amount of NaNO3 that was produced during the experiment. Explain how you determined this amount. (5 points)
Answers
NaCl is the limiting reactant and 9 moles of NaNO3 is obtained.
9 moles of NaNO3 is obtained, Balanced chemical reaction: Al(NO3)3 + 3NaCl 3NaNO3 + AICI3. From the reaction, it is seen that the limiting reactant yields the least amount of NaNO3.1 mole of Al(NO3)3 yields 3 moles of NaNO3. 4 moles of Al(NO3)3 yields 4 * 3/1 = 12 moles of NaNO3 Also,3 moles of NaCl yields 3 moles of NaNO3, 9 moles of NaCl yields 9 * 3/3 = 9 moles of NaNO3. Hence, NaCl is the limiting reactant and 9 moles of NaNO3 is obtained. The chemical equation is thought to be balanced when there are no inequalities. Every element in this example now has an equatable number of atoms on both the product and reactant sides. A balanced reaction has the same number of items on both sides of the equation. In a chemical reaction, the stoichiometric correlation is the number written in front of atoms, ions, and molecules to balance this same amount of each component on both the reactants and the products sides of the equation.
Learn more about chemical reaction here:
https://brainly.com/question/15355912
#SPJ4
what is the mass of 3.01x1023 atoms of iron(atomic mass of fe=56)
Answers
Answer:
mass=279grams
Explanation:
GIVEN DATA
number of atoms=3.01×10^23
avogadro's number=6.022×10^22
molar mass of iron=56g/moles
TO FIND
mass in gram of iron=?
SOLUTION
by using the formula
mass in gram=(number of atoms÷avogdro's number)×molar mass
mass=(3.01×10^23÷6.022×10^23)×56
mass=0.499×56
mass=27.9grams=28 grams
An FM radio station broadcasts at a frequency of 107.9 Hz. What is the
wavelength of the radio signal
Answers
Answer:
2.78035217*10^6 m
Explanation:
C = v
3.0e8 = (107.9)
= 2.78035217*10^6 m
What is the distance of a soccer player who runs the length of the pitch (20m)and then returns to the middle?
Answers
The distance of a soccer player who runs the length of the pitch (20m) and then returns to the middle is 30m.
Distance and displacement are two quantities that seem to mean the same but are distinctly different with different meanings and definitions. Distance is the measure of “how much ground an object has covered during its motion” while displacement refers to the measure of “how far out of place is an object.” In this article, let us understand the difference between distance and displacement.
Distance is the total movement of an object without any regard to direction. We can define distance as to how much ground an object has covered despite its starting or ending point.
In the question, The length of full pitch is 20m. So to cover full pitch one time the distance would be 20m and then the half pitch, the distance would be 10m. Total distance would be 30m.
Learn more about distance, here:
https://brainly.com/question/30510042
#SPJ1
chloride per milliliter (MW of CaCl2 = 147) [Round to the nearest whole number 5. What weight of magnesium chloride (MgCl2, formula weight = 95.3) is required to prepare 200 ml solution that is 5.0 mi
Answers
The weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
To calculate the weight of magnesium chloride (\(MgCl_{2}\)) required to prepare a 200 ml solution that is 5.0 M, we need to use the formula: Weight (in grams) = Volume (in liters) × Concentration (in moles/liter) × Molecular Weight (in grams/mole)
First, we convert the volume from milliliters to liters by dividing it by 1000: Volume = 200 ml ÷ 1000 = 0.2 L. Next, we multiply the volume, concentration, and molecular weight: Weight = 0.2 L × 5.0 mol/L × 95.3 g/mol = 47.65 grams
Rounding to the nearest whole number, the weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
This calculation ensures that the desired concentration is achieved by accurately measuring the appropriate amount of magnesium chloride, taking into account its molecular weight and the desired volume of the solution.
To know more about magnesium chloride, refer here:
https://brainly.com/question/30671024#
#SPJ11
Which element is the most reactive?sodiumnickelcarbonoxygen.
Answers
Answer:
The answer is sodium which is the element is the most reactive.
i need help from the problem below
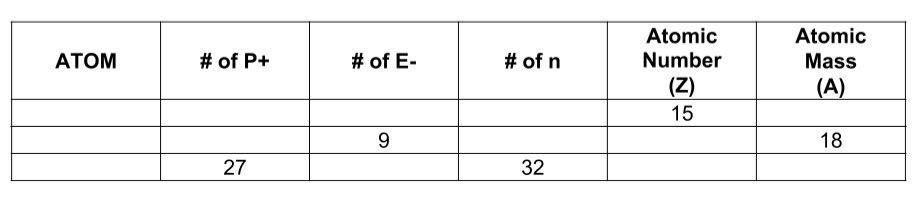
Answers
Answer:
I won't say I'm confident with this because I'm not sure if the second one is even correct, but I hope I helped you and didn't make things worse lol.

Calculate the mass of the 4He nucleus in atomic mass units by subtracting the mass of two electrons from that of a neutral 4 He atom. (See Appendix D, and give four decimal places.) The procedure suggested for part (a) is theoretically incorrect because we have neglected the binding energy of the electrons. Given that the energy needed to pull both electrons far away from the nucleus is 80 eV, is the correct answer larger or smaller than implied by part (a), and by how much (in u)? In stating the mass of the nucleus, about how many significant figures can you give before this effect would show up?
Answers
The correct answer should be larger than implied by part (a) by \(0.0859 \times 10^{(-3)\) u.
What is atomic mass?The atomic mass (also known as atomic weight) of an element represents the average mass of atoms of that element, taking into account the different isotopes and their abundances. It is typically expressed in atomic mass units (u).
The atomic mass of an element can be found on the periodic
To calculate the mass of the 4He nucleus in atomic mass units (u), we need to subtract the mass of two electrons from that of a neutral 4He atom. Let's proceed with the calculations:
According to Appendix D, the atomic mass of a neutral 4He atom is approximately 4.0026 u. We'll subtract the mass of two electrons to obtain the mass of the nucleus.
The atomic mass of an electron is approximately 0.00054858 u (as listed in Appendix D). Therefore, the mass of two electrons is:
2 * 0.00054858 u = 0.00109716 u
Subtracting this value from the mass of a neutral 4He atom:
4.0026 u - 0.00109716 u = 4.0015 u
This is the mass of the 4He nucleus in atomic mass units according to the incorrect calculation in part (a), which neglected the binding energy of the electrons.
However, we are given that the energy needed to pull both electrons far away from the nucleus is 80 eV. This energy corresponds to the binding energy of the electrons, which contributes to the mass of the system.
To convert 80 eV to atomic mass units (u), we use the conversion factor given in Appendix D: 1 u = 931.5 MeV/c².
80 eV is equal to 80 eV / (931.5 MeV/c²) = \(0.0859 \times 10^{(-3)\) u.
Therefore, the correct answer should be larger than implied by part (a) by \(0.0859 \times 10^{(-3)\) u.
In terms of significant figures, it's important to note that the binding energy of the electrons introduces an uncertainty in the mass calculation. The energy given (80 eV) is only specified to two significant figures. As a result, the mass of the nucleus should also be reported with a similar level of precision to reflect this uncertainty.
Learn more about atomic mass
https://brainly.com/question/29117302
#SPJ4
Sugar turns into black mass on heating Give reason
need fast
Answers
The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot. hope this helps
Sugar is made of carbon, hydrogen, and oxygen atoms. When heated over a candle, these elements react with the fire to turn into a liquid. The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot.
Predict the major products formed when benzene reacts with the following reagents. (a). tert-butyl bromide, ALCI3 (b) bromine + a nail (c) iodine + HNO3 (d) carbon monoxide, HCI, and AICI3/CuCl (e) nitric acid + sulfuric acid.
Answers
The major products formed when benzene reacts with the following reagents are :
(a) tert-butylbenzene
(b) bromobenzene
(c) mixture (ortho-nitrobenzene, meta-nitrobenzene, para-nitrobenzene)
(d) benzaldehyde
(e) nitrobenzene
(a) The major product formed when benzene reacts with tert-butyl bromide and \(AlCl_3\) is tert-butylbenzene.
(b) The major product formed when benzene reacts with bromine and a nail (iron) is bromobenzene.
(c) The major product formed when benzene reacts with iodine and \(HNO_3\) is a mixture of ortho-nitrobenzene, meta-nitrobenzene, and para-nitrobenzene.
(d) The major product formed when benzene reacts with carbon monoxide, HCl, and \(AlCl_3\)/CuCl is benzaldehyde.
(e) The major product formed when benzene reacts with nitric acid and sulfuric acid is nitrobenzene.
To learn more about bromobenzene follow the link:
https://brainly.com/question/32141237
#SPJ4
A. How well do the continents fit together
Answers
Answer:
The shapes of continents fit together like a puzzle.
Explanation:
Just look at the east coast of South America and the west coast of Africa—it's almost a perfect fit! Identical rocks have been found on different continents. These rocks formed millions of years ago before the continents separated.
When lithium metal reacts with fluorine gas it forms the ionic compound lithium fluoride (LiF). What is the correct electron configurations of the ions formed
Answers
Answer:
The electronic configuration of ions formed are:
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Explanation:
Lithium metal, Li will lose 1 electron to lithium ion Li+. Chlorine atom, Cl will receive the 1 electron to form the chloride ion Cl- as shown by the following equation below:
Li —> Li+ + e
Cl+ e —> Cl-
Combine both equation
Li + Cl + e —> Li+ + Cl- + e
Cancel out 'e'
Li + Cl —> Li+ Cl-
Thus, we can write the electronic configuration for the reaction as follow:
Before reaction:
Li (3) => 1s2 2s1
Cl (17) => 1s2 2s2 2p6 3s2 3p5
After reaction
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Therefore, the electronic configuration of the ions formed are:
Li+ (2) => 1s2
Cl- (18) => 1s2 2s2 2p6 3s2 3p6
Does boron have 2 valence electrons.
Answers
in a multielectron atom, can we predict unambiguously whether the 4s orbital is lower or higher in energy than the 3d orbitals? explain your answer.
Answers
The 4s orbitals are filled first because, according to our theory, they have a lower energy than the 3d orbitals. We are aware that the 4s electrons are lost first when an atom ionizes.
The highest energy level electrons that are lost first are those that are most far from the nucleus' influence. A higher energy must therefore exist in the 4s orbital than in the 3d orbitals.
The electron is a subatomic particle with the symbol e or with an electric charge of one elementaryly negative charge. Due to their lack of known components or substructure, electrons, which are part of the first generation of the lepton particle family, are typically considered to be elementary particles.
Learn more about electron here
https://brainly.com/question/18914648
#SPJ4
How to we know how many neutrons are in the atoms we draw models for ? (We represent average atoms, not ions or isotopes)
Answers
Answer:
Atoms is the smallest unit of a chemical element and consist of three main components protons, neutrons, and electrons.
The number of a neutron is based on the difference between the mass number of the atom (M) and the atomic number (Z). every isotope of an element has a different number of neutron.
But in a neutral atom or average atom, the number of neutrons is equal to the number of protons and the number of electron.
Example of the number of neutrons in an average atom: In Nitrogen-14, the atomic number and the number of protons is 7, it means the number of neutrons will also 7.
a mixture of ch4 (g) and c2h6 (g) has a total pressure of 0.53 atm. just enough o2 was added to the mixture to bring about it's complete combustion to co2 (g) and h2o (g). the total pressure of the two product gases is found to be 2.2 atm. assuming constant volume and temperature, find the mole fraction of ch4 in the original mixture.
Answers
the mole fraction of \(CH_4\) in the original mixture is 0.73 = 73%.
How do we calculate?Total pressure of the mixture = 0.53 atm
Total pressure of the product gases = 2.2 atm
The combustion equation for \(CH_4\) is given as :
\(CH_4\)(g) + \(2O_2\)(g) -> \(CO_2\)(g) + \(2H_2O\)(g)
We then apply Dalton's law of partial pressures, and write
Pressure of \(CO_2\) + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = Pressure of product
x + Pressure of \(H_2O\) = 2.2 atm
the mole fraction and the partial pressure relationship is :
2x = Pressure of \(H_2O\)
x + 2x = 2.2 atm
3x = 2.2 atm
x = 2.2 atm / 3
x = 0.73
Learn more about mole fraction at;
https://brainly.com/question/29065092
#SPJ4
the pka of phosphoric acid is 7.21. what is the useful buffering range of this acid and its conjugate base? briefly, explain.
Answers
To answer your question, the useful buffering range of phosphoric acid and its conjugate base is approximately between pH 6.21 to 8.21.
This is because the pKa of phosphoric acid is 7.21, which means that at pH values below 6.21, most of the acid will be in its protonated form (H3PO4) and at pH values above 8.21, most of it will be in its deprotonated form (H2PO4-). However, within this buffering range, there will be a relatively equal distribution of both the protonated and deprotonated forms,
which allows for the acid-base pair to act as an effective buffer. The buffer capacity is the highest at pH = pKa and decreases as the pH moves away from pKa. The conjugate base of phosphoric acid is H2PO4-, and it acts as the base in the buffer system. In summary, the useful buffering range of phosphoric acid and its conjugate base is between pH 6.21 to 8.21, which allows for the acid-base pair to act as an effective buffer.
To know more about phosphoric acid visit:
https://brainly.com/question/30489231
#SPJ11
Can you use volume alone to predict whether an object will sink or float? Explain your answer
Answers
Answer:
Can volume alone be used to predict whether an object will sink or float? -No, you need both mass and volume to find its density to see if it can float.
A gas has a solubility of 0.086 g/l at a pressure of 3.5 atm. At what pressure would the solubility be at 2.3 g/L?
Answers
The pressure at which the the gas has a solubility of 2.3 g/L is 93.95 atm
How to find the pressure?To find the pressure at which the solubility would be 2.3 g/L, we need to use equation for Henry's law, which is written as:
S₁/P₁ = S₂/P₂
Where the variables in that equation are:
S₁ = Initial solubilityP₁ = Initial pressureS₂ = Final solubilityP₂ = Final pressureWe know the values of:
S₁ = 0.086 g/L
P₁ = 3.5 atm
S₂ = 2.3 g/L
Solving the equation for P₂:
S₁/P₁ = S₂/P₂
P₂ = (S₂ * P₁) / S₁
Now evaluate it to get:
P₂ = (2.3 g/L * 3.5 atm) / 0.086 g/L
P₂ = 93.95 atm
Learn more about solubility at:
https://brainly.com/question/23946616
#SPJ1
If a liquid has a density of 0.785, then any object with _____ density will float.
More
Less
Answers
How many mL of ethanol would you
add to water to make 1750 mL of a
40.0% ethanol solution (v/v%)?
Answers
Answer:
700 mL of ethanol would be added
Explanation:
Given data:
Volume of ethanol added to make 40% v/v solution = ?
Total volume of solution = 1750 mL
Solution:
We will calculate the 40% 1750 mL which will be the volume of ethanol added to the water to make 40%v/v solution.
(40/100)× 1750 mL
0.4 × 1750 mL
700 mL
700 mL of ethanol would be added.
why are fish lucky that water particles expand as they hit a temperature of 0°c?
Answers
Answer:
it is a result of hydrogen bonds present within water molecules.
Explanation:
when the water is transformed to ice at zero degrees Celsius, the water molecules are in crystal lattice in a structure that has a lot of empty space around each molecule.
Two scientists are investigating the properties of water. In one experiment, they notice that water breaks down to hydrogen and oxygen gas during a chemical reaction. Which of the following does this experiment demonstrate
A. it demonstrates that a water molecule consists of two hydrogen atoms and one oxygen atom.
B. it demonstrates that water consists of atoms
C. it demonstrates that water is a compound
D. it demonstrates that water is an element
Answers
Answer:
A
Explanation:
a gas sample is collected in a balloon. what happens to the density of the gas sample as the sample is heated?
Answers
The density of the gas will decrease as we heat the gas.
Density of a system is defined by mass per unit volume. Density basically describes how compact or concentrated something is. For example, suppose you have two boxes, one is large and one is small. However, they both weigh the same. But we see that the small box has a higher density than the large box. So, density also tells how concentrated or crowded something is.
Considering the above given case, when we take a gas sample in a balloon and then when we start to heat this sample, due to hear we can see that the gas molecules will try to move more far from each other and therefor the density of the system will decrease. This same concept is been used in hot air balloons too. When we heat the balloon the density of the gas will decrease and therefor the balloon floats in the air.
To know more about density
https://brainly.com/question/15164682
#SPJ4
Describe at least two ways that carbon can get from the atmosphere to the hydrosphere.
Answers
Answer:
One way is that carbon dioxide automatically dissolves in cold ocean surface waters anyway so large bodies of water already have a concentration of carbon dioxide. Another way is when carbon dioxide dissolves in rainwater which later reacts with minerals in rocks. These rocks then get carried away by rivers or streams into bigger bodies of water and the carbon is transported through the minerals in the rocks.
please help me
16 1 point What is the decay rate of a sample of Oxygen-21 if the sample has 8.31x1017 atoms and a decay constant of 0.203/s? 4.09x1018Bq 1.69x10¹7Bq 0.203Bq 2.44x10-1⁹Bq Previous
Answers
decay rate of approximately 1.69x10^17 Bq (becquerels),
The decay rate of a radioactive sample is determined by the number of radioactive atoms present and the decay constant, which represents the probability of decay per unit of time.
To calculate the decay rate, we multiply the number of atoms in the sample by the decay constant. In this case, the sample has 8.31x10^17 atoms and a decay constant of 0.203/s. Multiplying these values gives a decay rate of approximately 1.69x10^17 Bq (becquerels), which represents the number of decays per second in the sample.
Learn more about Oxygen here : brainly.com/question/13905823
#SPJ11
Balance each of the following examples of heterogeneous equilibria and write each reaction quotient, Qc:(c) N₂O₅(s) ⇄ NO₂(g) + O₂(g)
Answers
When we balance this equation
N₂O₅(s) ⇄ NO₂(g) + O₂(g)
We will get
2N₂O₅(s) ⇄ 4NO₂(g) + O₂(g)
Balancing this equation
N₂O₅(s) ⇄ NO₂(g) + O₂(g)
We have to balance the number of N
N₂O₅(s) ⇄ 2NO₂(g) + O₂(g)
We have to balance the number of O
2N₂O₅(s) ⇄ 4NO₂(g) + O₂(g)
The balanced equation is
2N₂O₅(s) ⇄ 4NO₂(g) + O₂(g)
The reaction quotient will be
Qc = [product] / [reactant]
Qc = [NO₂(g) + O₂(g)] / [ N₂O₅(s)]
To learn more about balanced equation click the given link
https://brainly.com/question/14237111
#SPJ4
what is fractional distinction?
Answers
Answer:
ANSWERFractional distillation is a technique used to separate miscible liquids that have boiling point difference of less than 25 ºC. The only difference in apparatus between simple and fractional distillation methods is the use of a fractionating column used during fractional distillation.\(\sf\large\underline\purple{Fractional\: Distillation:-}\)
Fractional distillation is a type of distillation which involves the separation of miscible liquids.\(\rightarrow\)The process involves repeated distillations and condensations and the mixture is usually separated into component parts. The separation happens when the mixture is heated at a certain temperature where fractions of the mixture start to vaporize.
\(\rightarrow\)The basic principle of this type of distillation is that different liquids boil and evaporate at different temperatures. So when the mixture is heated, the substance with lower boiling point starts to boil first and convert into vapours.
\(\sf\underline\green{Applications\: Of\:Fractional\: Distillation:-}\)
Fractional distillation is used for the purification of water as well as separating acetone and water.Fractional distillation is used in several industries like oil refineries and chemical plants mainly for purification and separation of many organic compounds.Fractional distillation is also used for the separation of (liquefied) air. Components like liquid nitrogen and oxygen as well as concentrated argon are obtained.Distillation is used in the production of high-purity silicon from chlorosilanes. The silicon is widely used in semiconductors.________________________________
Hope it helps you:)

Titanium is a transition metal used in many alloys because it is extremely strong and lightweight. Titanium tetrachloride (TiCl4) is extracted from titanium oxide using chlorine and coke (carbon). TiO2(s) + C(s) + 2 Cl2(g) TiCl4(s) + CO2(g) (a) What mass of Cl2 gas is needed to react with 1.60 mol TiO2? g (b) What mass of C is needed to react with 1.60 mol of TiO2? g (c) What is the mass of all the products formed by reaction with 1.60 mol of TiO2? g
Answers
Answer:
a) 226.6 grams of Cl₂
b) 19.2 grams of C
c) 303.2 grams of TiCl₄ and 70.4 grams of CO₂
Explanation:
The balanced chemical reaction is the following:
TiO₂(s) + C(s) + 2 Cl₂(g) → TiCl₄(s) + CO₂(g)
(a) What mass of Cl₂ gas is needed to react with 1.60 mol TiO₂?
From the chemical equation, 1 mol of TiO₂ reacts with 2 moles of Cl₂. So, the stoichiometric ratio is 2 mol Cl₂/1 mol TiO₂. We multiply this ratio by the moles of TiO₂ we have to calculate the moles of Cl₂ we need:
1.60 mol TiO₂ x 2 mol Cl₂/1 mol TiO₂ = 3.2 mol Cl₂
Now, we convert from moles to mass by using the molecular weight (MW) of Cl₂:
MW(Cl₂) = 35.4 g/mol x 2 = 70.8 g/mol
mass of Cl₂= 3.2 mol x 70.8 g/mol = 226.6 g
Therefore, 226.6 grams of Cl₂ are needed to react with 1.6 mol of TiO₂.
(b) What mass of C is needed to react with 1.60 mol of TiO₂?
From the chemical equation, 1 mol of TiO₂ reacts with 1 moles of C(s). So, the stoichiometric ratio is 1 mol C/1 mol TiO₂. We multiply this ratio by the moles of TiO₂ we have to calculate the moles of C(s) we need:
1.60 mol TiO₂ x 1 mol C(s)/1 mol TiO₂ = 1.60 mol C(s)
So, we convert the moles of C(s) to grams as follows:
MW(C) = 12 g/mol
1.60 mol x 12 g/mol = 19.2 g C(s)
Therefore, a mass of 19.2 grams of C is needed to react with 1.60 mol of TiO₂.
(c) What is the mass of all the products formed by reaction with 1.60 mol of TiO₂?
From the chemical equation, we can notice that 1 mol of TiO₂ produces 1 mol of TiCl₄ and 1 mol of CO₂. So, from 1.60 moles of TiO₂, 1 mol of each product will be produced:
1 mol TiO₂/1 mol TiCl₄ ⇒ 1.60 mol TiO₂/1.60 mol TiCl₄
1 mol TiO₂/1 mol CO₂ ⇒ 1.60 mol TiO₂/1.60 mol CO₂
Finally, we convert the moles to grams by using the molecular weight of each compound:
MW(TiCl₄) = 47.9 g/mol Ti + (35.4 g/mol x 4 Cl) = 189.5 g/mol
1.60 mol x 189.5 g/mol = 303.2 g
MW(CO₂) = 12 g/mol C + (16 g/mol x 2 O) = 44 g/mol
1.60 mol x 44 g/mol = 70.4 g
Therefore, from the reaction of 1.60 mol of TiO₂ are formed 303.2 grams of TiCl₄ and 70.4 grams of CO₂.