what percent of glucose, C6H12O6, is carbon
Answers
Answer:
Explanation:
Definition of Percentage by weight
It is defined as the mass percent composition of an element in a compound.
It can be abbreviated as w/W %
Percentage of carbon by weight in glucose:
Let us calculate the mass of glucose= 6 x mass of C + 12 x mass of H + 6 x mass of O
= 6 x 12 + 12 x 1 + 6 x 16
= 180 g
Total mass of carbon = 6 x 12 = 72 g
Therefore the percentage of carbon by weight in glucose is:
40 percentage
Conclusion: Glucose ( C 6 H 12 O 6 ) contains 40 percentage of carbon by weight.
Conclusion: Glucose
contains 40percente of carbon by weight.
Related Questions
A 13.3 mg sample of a protein is dissolved in water to make a 24.7 mL solution. The osmotic pressure is 0.59 mmHg at 28oC. What is the molar mass of the protein
Answers
The molar mass of the protein is approximately 13,253 g/mol.
The molar mass of the protein can be determined using the formula for osmotic pressure and the ideal gas law.
The osmotic pressure (π) is given as 0.59 mmHg, which needs to be converted to atm for consistency. 1 mmHg is equivalent to 0.001315 atm.
The volume (V) of the solution is given as 24.7 mL, which needs to be converted to liters by dividing by 1000.
The temperature (T) is given as 28°C, which needs to be converted to Kelvin by adding 273.15.
The ideal gas law equation is π = (n/V)RT, where n is the number of moles, V is the volume, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation, we have n = (πV) / (RT).
Substituting the given values, we get n = (0.59 × 0.001315 × 24.7) / (0.0821 × 301.15).
Simplifying the equation gives n = 0.001003 mol.
To calculate the molar mass (M) of the protein, we divide the mass (m) of the protein by the number of moles (n). The mass of the protein is given as 13.3 mg, which needs to be converted to grams by dividing by 1000.
Therefore, M = (13.3 / 1000) / 0.001003 = 13,253 g/mol.
Hence, the molar mass of the protein is approximately 13,253 g/mol.
To know more about Molar mass, visit:
https://brainly.com/question/12127540
#SPJ11
if
half life of C -14 is 5700 years. how many years pass a sample
decays from an activity of 1050 to an activity of 205
Answers
It will take approximately 18197 years for the sample of C-14 to decay from an activity of 1050 to an activity of 205.
The question is asking for the number of years that will pass before a sample of C-14 decays from an activity of 1050 to an activity of 205. Given that the half-life of C-14 is 5700 years, we can use the formula for exponential decay to solve for the time required. The formula is:
N = N₀ (1/2)^(t/t₁/₂)
where:
N = final amount
N₀ = initial amount
t = time elapsed
t₁/₂ = half-life
We can rearrange the formula to solve for t:
t = t₁/₂ (ln(N₀/N)) / ln(1/2)
Using the given values, we have:
N₀ = 1050
N = 205
t₁/₂ = 5700
Substituting into the formula:
t = 5700 (ln(1050/205)) / ln(1/2)
t ≈ 18197 years (rounded to the nearest year)
Learn more about C-14
https://brainly.com/question/1500129
#SPJ11
It will take approximately 18197 years for the sample of C-14 to decay from an activity of 1050 to an activity of 205.
The question is asking for the number of years that will pass before a sample of C-14 decays from an activity of 1050 to an activity of 205. Given that the half-life of C-14 is 5700 years, we can use the formula for exponential decay to solve for the time required. The formula is:
N = N₀ (1/2)^(t/t₁/₂)
where:
N = final amount
N₀ = initial amount
t = time elapsed
t₁/₂ = half-life
We can rearrange the formula to solve for t:
t = t₁/₂ (ln(N₀/N)) / ln(1/2)
Using the given values, we have:
N₀ = 1050
N = 205
t₁/₂ = 5700
Substituting into the formula:
t = 5700 (ln(1050/205)) / ln(1/2)
t ≈ 18197 years (rounded to the nearest year)
Learn more about C-14
brainly.com/question/1500129
#SPJ11
A lake has been infected by some type of new algae that is unknown. Every single day the amount of surface area that the algae takes up doubles. Day 1 has a certain amount, day 2 it is 2x that amount. It takes 87 days for the entire lake to be overrun by this new algae. How many days does it take to cover half of the lake? Show your work and explain your thinking. In science we need to be able to justify our answers.
hint: It is not the "obvious" answer.
Answers
Answer:
It takes 86 days take to cover half of the lake
Explanation:
In the day #1, the amount of the algae is X,
In the day #2 is 2X
In the day #3 is 2*2*X = X*2²
...
In the day #n the amount of the algae is X*2^(n-1)
Assuming X = 1m³. In the day 87, the area infected was:
1m³*2^(87-1)
7.74x10²⁵m³ is the total area of the lake
the half of this amount is 3.87x10²⁵m³
The time transcurred is:
3.87x10²⁵m³ = 1m³*2^(n-1)
Multiplying for 5 in each side:
ln (3.87x10²⁵) = ln (2^(n-1))
58.9175 = n-1 * 0.6931
85 = n-1
86 = n
It takes 86 days take to cover half of the lakewhich biological molecules are composed of a long hydrocarbon chain with a carboxyl (cooh) group at one end?
Answers
Fatty acids are composed of a long hydrocarbon chain with a carboxyl (cooh) group at one end.
Long-chain hydrocarbons with a carboxylic acid functional group are fatty acids. They are hydrophobic due to their very lengthy nonpolar hydrocarbon chains. Unsaturated fatty acids are those with double bonds; saturated fatty acids are those without double bonds.
It is made up of a straight chain with an even number of carbon atoms, hydrogen atoms along the length of the chain, a carboxyl group (COOH) at one end, and hydrogen atoms at both ends. It is an acid (carboxylic acid) because of the carboxyl group.
Due to its tetravalent nature, carbon can only connect to other hydrocarbon chains at the ends of the parent chain.
To know about hydrocarbon
https://brainly.com/question/30630718
#SPJ4
Please help ASAP.....please
would you choose to buy a soft drink in a plastic bottle or an aluminium can? Explain your answer.(3 marks)
Answers
Answer:
In a bottle.
Explanation:
I would like soda in a bottle because you don't get soda stuck in the aluminum can like you do a bottle. And it's also way more efficient because bottles have caps that you can screw on later, but aluminum doesn't.
list the formula and names for all functional groups; give their significance and location in various organic molecules we have studied.
Answers
There are several functional groups that are found in organic molecules, such as hydroxyl group, carbonyl group, carboxyl group, amino group and phosphate group.
One important functional group is the hydroxyl group, which is represented by the formula -OH. This group is found in alcohols, such as ethanol, and is important for their solubility in water and ability to form hydrogen bonds. Another functional group is the carbonyl group, which is represented by the formula -C=O. This group is found in aldehydes and ketones, and is important for their reactivity in chemical reactions. The carboxyl group, represented by the formula -COOH, is found in carboxylic acids and is important for their acidic properties.
The amino group, represented by the formula \(-NH_2\), is found in amino acids and is important for their ability to form peptide bonds and create proteins. Finally, the phosphate group, represented by the formula \(-PO_4\), is found in nucleotides and is important for their role in DNA and RNA synthesis.
To learn more about organic molecules click here https://brainly.com/question/28843074
#SPJ11
a sample of an ideal gas has a volume of 3.50 l3.50 l at 12.00 ∘c12.00 ∘c and 1.40 atm.1.40 atm. what is the volume of the gas at 20.80 ∘c20.80 ∘c and 0.986 atm?
Answers
The volume of a fixed amount of gas is directly proportional to its absolute temperature So 5.12 L.
A really perfect gasoline is a theoretical gas composed of many randomly transferring factor debris that is not difficult to interparticle interactions. the proper gas idea is beneficial as it obeys an appropriate fuel regulation, a simplified equation of state, and is amenable to analysis beneath statistical mechanics.
Calculation:-
Volume of ideal gas = 3.50 L
Temperature = 12⁰C = 285 K
Pressure = 1.40 atm
Temperature 2 = 20.80 ⁰C = 293.80 K
Pressure = 0.986 atm
New Volume V 2 = PVT/TP
= 1.40 × 3.50 × 293.80 / 285 × 0.986
= 5.12 L
New volume is 5.12 L.
A really perfect fuel is described as a fuel that obeys gas laws at all conditions of strain and temperature. best gases have speed and mass. They do now not have volume ideal fuel, additionally referred to as ideal fuel, a fuel that conforms, in physical behavior, to a specific idealized relation between strain, extent, and temperature called the appropriate, or trendy, gas regulation.
The gas particles have negligible volume. The fuel particles are similarly sized and do not have intermolecular forces of attraction or repulsion with different gasoline particles. The gasoline debris moves randomly in settlement with Newton's legal guidelines of motion. The gas debris has perfect elastic collisions without an electricity loss.
Learn more about ideal gas here:-https://brainly.com/question/14889179
#SPJ4
Why is carbon special?
Answers
Answer:
they can bond together to form very long, durable chains that can have branches or rings of various sizes and often contain thousands of carbon atoms. Silicon and a few other elements can form similar chains; but they are generally shorter, and much less durable.
Explanation:
help does anyone know this

Answers
what is the mass of electron
Answers
Explanation:
The rest mass of the electron is 9.1093837015 × 10−31 kg, which is only 1/1,836the mass of a proton. An electron is therefore considered nearly massless in comparison with a proton or a neutron, and the electron mass is not included in calculating the mass number of an atom.
Answer: The Mass of an electron is 9.1093837 x 10^-31 kgs
Explanation:
Do M&M's melt in your hand?
Answers
Answer:
yea hold them in your hand for a while and your hand will look like a rainbow
Explanation:
Which reaction occurs at the cathode of a galvanic cell that has the following: an aluminum electrode in an electrolyte with aluminum ions and a zing electrode in an electrolyte with zinc ions? The reduction potential for the
reduction of A13+ = -1.68 V. The reduction potential for the reduction of Zn2+
-0.76 V.
O A. Zn2+ (ag) + 28
-> Zn(s)
O B. Al(s) - Al$+(34) + 3e
O C. Zn(s) -> Zn2+ (ag) + 2€
O D. AlS*(a9) + 36 -> Al(s)

Answers
The reaction that occurs at the cathode of the illustrated galvanic cell is Zn(s) -> Zn2+ (aq) + 2e-. Option C
What is reduction potential?The reaction that occurs at the cathode of a typical galvanic cell depends on the relative reduction potentials of the two half-reactions involved.
In this case, the reduction potential for the reduction of Al3+ (-1.68 V) is more negative than the reduction potential for the reduction of Zn2+ (-0.76 V). This means that the reduction of Al3+ requires more energy than the reduction of Zn2+.
Since the cathode is the site of reduction, the half-reaction with the more positive reduction potential (i.e., the reduction of Zn2+) will occur at the cathode.
Therefore, the correct answer is Zn(s) -> Zn2+ (aq) + 2e-.
More on reduction potential can be found here: https://brainly.com/question/8739272
#SPJ1
True or false: the moment of inertia of a hoop that has a mass and a radius is greater than the moment of inertia of a disk that has the same mass and radius.
Answers
It is true that the moment of inertia of a hoop that has a mass and a radius is greater than the moment of inertia of a disk that has the same mass and radius.
Lower moments of inertia signify that only small forces are required to create a rotation, whereas higher moments of inertia mean that greater force must be used. The masses with the largest moment of inertia are those that reside furthest from the axis of rotation.
Due to the fact that a ring's center of mass is located on an axis that is perpendicular to its plane and passes through it, a ring has a higher moment of inertia than that of a circular disc of equal mass and radius. Because all of a ring's mass has been concentrated at the rim, which is furthest from the axis, which has a greater moment of inertia.
Therefore, the given statement is true.
To know more about moment of inertia
https://brainly.com/question/23877963
#SPJ4
how much space in liters (l) would 40.4 g of neon (ne) gas occupy at stp (standard temperature and pressure)?
Answers
To determine how much space 40.4 g of neon gas would occupy at STP, we first need to convert the mass of neon to the number of moles. The molar mass of neon is approximately 20.18 g/mol.
Number of moles of neon = mass of neon / molar mass of neon
Number of moles of neon = 40.4 g / 20.18 g/mol
Number of moles of neon = 2.0 mol
So, we have 2.0 moles of neon gas. At STP, 1 mole of any ideal gas occupies a volume of 22.4 liters. Therefore, 2.0 moles of neon gas will occupy:
Volume of neon gas = number of moles of neon x molar volume at STP
Volume of neon gas = 2.0 mol x 22.4 L/mol
Volume of neon gas = 44.8 L
Therefore, 40.4 g of neon gas would occupy 44.8 liters of volume at STP.
What is STP?STP stands for Standard Temperature and Pressure. It is a standard set of conditions used in chemistry and physics for the measurement and comparison of gases.
The standard temperature for STP is 0 degrees Celsius (273.15 Kelvin). The standard pressure for STP is 1 atmosphere (atm), which is equivalent to 101.325 kilopascals (kPa) or 760 millimeters of mercury (mmHg).
Learn about STP here https://brainly.com/question/29796637
#SPJ1
Convert the following number from scientific notation to standard notation:
7.80 x 105
Answers
Answer:
Call of balls massive sack
Explanation:
Yoooo
Which of the following multistep reaction pathways will give a higher yield and why? Pathway #1, because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways. Pathway #2, because there is much steric hindrance for secondary substrates than for primary substrates in the SN2 reaction, which is the first mechanism in each of the pathways. Both pathways will give the same yield.
Answers
Pathway #1 will give a higher yield because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways.
The SN2 reaction proceeds via a concerted, bimolecular, one-step mechanism, where the nucleophile attacks the electrophilic carbon center, and the leaving group is simultaneously displaced. In pathway #1, the SN2 reaction involves a primary substrate with a less hindered leaving group, which will react faster and give a higher yield.
On the other hand, pathway #2 involves a secondary substrate with a more hindered leaving group, which will react slower and give a lower yield. Hence, pathway #1 is more efficient because of the higher reactivity of the primary substrate and the less steric hindrance in the reaction mechanism.
Therefore, the pathway #1 will give a higher yield than pathway #2 in a multistep reaction pathway because there is much less steric hindrance for primary substrates than for secondary substrates in the SN2 reaction, which is the first mechanism in each of the two pathways.
Learn more about leaving group here:
https://brainly.com/question/31831803
#SPJ11
be sure to answer all parts. a chloride of silicon contains 79.1 mass % cl. (a) what is the empirical formula of the chloride? (b) if the molar mass is 269 g/mol, what is the molecular formula?
Answers
The empirical formula of given chloride of silicon is SiCl5 and its molecular formula is Si6Cl30
(a) The empirical formula of a compound represents the simplest whole number ratio of its constituent elements. To find the empirical formula of the chloride of silicon, we can use the percentage composition data.
First, convert the percentage composition of chlorine to grams:
79.1 g Cl / 100 g = 0.791 g Cl
Next, find the number of moles of chlorine:
0.791 g Cl / 35.5 g/mol = 0.022 mol Cl
Since the mass of the silicon is not specified, we can assume that the rest of the mass of the compound is silicon. To find the number of moles of silicon, we subtract the moles of chlorine from the total number of moles:
1.000 mol - 0.022 mol = 0.978 mol Si
Next, divide both the moles of chlorine and silicon by the smallest number of moles, to obtain the simplest whole-number ratio:
0.022 mol Cl / 0.022 mol Cl = 1
0.978 mol Si / 0.022 mol Cl = 44.5
Since 44.5 is not a whole number, we multiply both the moles of chlorine and silicon by the same factor to obtain whole numbers:
0.022 mol Cl * 5 = 0.11 mol Cl
0.978 mol Si * 5 = 4.89 mol Si
Finally, the empirical formula of the chloride of silicon is: SiCl5
(b) The molecular formula of a compound represents the actual number of atoms of each element in a molecule of the compound. To find the molecular formula of the chloride of silicon, we can use the molar mass and the empirical formula.
Given that the molar mass is 269 g/mol, we can calculate the number of empirical formula units in a molecule of the compound:
269 g/mol / (44 g/mol Si + 5 x 35.5 g/mol Cl) = 6
Therefore, the molecular formula of the chloride of silicon is:
SiCl5 x 6 = Si6Cl30
The molecular formula of the chloride of silicon is six times the empirical formula, SiCl5, with a molar mass of 269 g/mol.
To know more about empirical formula, click here : https://brainly.com/question/1439914
#SPJ4
The density of gold 19.3g/cm3. if a sample of pure gold has a mass of 65.4g, what is the volume?
Answers
The volume of pure gold has a mass of 65.4g and a density of 9.3g/cm3 is 3.3886 cm3.
Solution :
∵ density = mass ÷ volume
⇒ volume = mass ÷ density
∴ volume of pure gold = (mass of gold) ÷ (density of gold)
= 65.4 ÷ 19.3
The volume of pure gold = 3.3886 cm3
Learn more about Density at :
brainly.com/question/14667456
14. A gas in a 400 ml flask exerts pressure of 81.0 kPa at 25.0°C. If the gas
was in a 250 ml container at a pressure of 101 kPa, the temperature would be
a) - 124 °C
b) - 41.2°C
c) 20.0°C
d) 232 °C
Answers
Answer: B would be the correct answer
Explanation: You will need to use P1V1/T1=P2V2/T2
So
P1= 81.0kPa /101 =0.799atm
V1=0.4L
T1=25.0C +273.15K= 298.15K
P2= 101kPa /101 = 1atm
V2=0.25L
T2= ?
T2=P2V2T1/P1V1
(1.0atm)(0.25L)(298.15K)/(0.799atm)(0.4L) =233.22K-273.15=-39.92C
explain why the troposphere has a larger total mass in the stratosphere even though the stratosphere so much bigger
Answers
Answer:
The troposphere is the lowest layer of Earth's atmosphere, and is also where nearly all weather conditions take place. It contains 75% of the atmosphere's mass and 99% of the total mass of water.
Explanation:
Rutherford's model of atom could not explain:
Select one:
a.
Intensive properties
b.
Physical properties
c.
Chemical properties
d.
Extensive properties
Answers
Rutherford's model of atom could not explain chemical properties as it did not make any mention as to how chemical changes take place.
What are chemical properties?These properties are defined as those properties which become evident during or after a chemical reaction after the identity of the substance is changed during chemical reaction.
These properties cannot be determined externally just by viewing the substance ,these change immensely after a substance undergoes a chemical change.These are used for identification of unknown substances and for building up chemical classifications.
The major chemical properties are flammability,toxicity,reactivity,acidity and heat of combustion.For a chemical property to be apparent , it is necessary that the structure of the substance is altered.
Learn more about chemical properties,here:
https://brainly.com/question/5186976
#SPJ1
What is the bond order of N2+? Express the bond order numerically. Is N2+ paramagnetic or diamagnetic? paramagnetic diamagnetic neither
Answers
Bond order of N2+ is 2.5. It is a diamagnetic substance.
Bond order is termed as the number of chemical bonds between a pair of the atoms. For example: In case of acetylene the bond order between the two carbon atoms is 3, in diatomic nitrogen the bond order is 3, and the C-H bond order is 1.
The bond order of N2+ is 2.5.
Bond order = 1 / 2[Nb - Na] Where, Nb = no. of electrons in bonding molecular orbital and Na = number of electrons in antibonding molecular orbital.
Bond order = 9-4 / 2
= 2.5
N2+ is diamagnetic in nature because they do not have any unpaired electrons they are having 14 electrons.
To know more about bond order here
https://brainly.com/question/29853110
#SPJ4
Fritz Haber, a German chemist, discovered a way to synthesize ammonia gas (NH3) by combining hydrogen and nitrogen gases at extremely high temperatures and pressures. a. Write the balanced equation for this reaction. b. If 10 kg of nitrogen combines with excess hydrogen at 550°C and 250 atm, what volume of ammonia gas is produced?
Answers
a. The balanced equation for the synthesis of ammonia gas is: N\(_{2}\) + 3H\(_{2}\) → 2NH\(_{3}\).
b. The volume of ammonia gas produced is approximately 1934.74 liters.
To determine the volume of ammonia gas produced, we need to use the ideal gas law equation, PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature.
Given:
Mass of nitrogen = 10 kg
Temperature = 550°C = 823 K
Pressure = 250 atm
First, we need to calculate the number of moles of nitrogen. The molar mass of nitrogen (N\(_{2}\)) is approximately 28 g/mol. Using the given mass of nitrogen (10 kg), we can convert it to grams (10,000 g) and then divide by the molar mass to get the number of moles (357.14 mol).
From the balanced equation for the synthesis of ammonia (N\(_{2}\) + 3H\(_{2}\) → 2NH\(_{3}\)), we know that 1 mole of nitrogen reacts to produce 2 moles of ammonia.
Therefore, the number of moles of ammonia produced is twice the number of moles of nitrogen, which is 2 * 357.14 = 714.29 mol.
Now, we can use the ideal gas law to calculate the volume of ammonia gas. Rearranging the equation, V = (nRT) / P, we can plug in the values:
V = (714.29 mol * 0.0821 L·atm/(mol·K) * 823 K) / 250 atm = 1934.74 L.
So, the volume of ammonia gas produced is approximately 1934.74 liters.
You can learn more about balanced equation at
https://brainly.com/question/11904811
#SPJ11
a claim about arrangements of electrons and properties within a family elements
Answers
A claim about the arrangements of electrons and properties within a family of elements is described below:
elements in the same family have the same number of outermost shell electronselements in the same family have similar chemical properties due to them having the same arrangements of electronsWhat are families of elements?Families of elements refer to elements that are found in the same group in the periodic table.
Elements that belong to the same family have the same arrangement of electrons.
The families of elements are found in the vertical columns knowns as groups. They have the same physical properties because they have the same number of e; electrons in their outermost shell.
For example, elements belonging to group 1 have one valence electron and show similarity in their chemical properties.
Learn more about families or groups of elements at: https://brainly.com/question/13870873
#SPJ1
how much calcium in gram is present in Ca(NO3)2 that contains 1.4 gram of Nitrogen
Answers
Answer:
28.57 grams are in (NO3)2
Explanation:
4) Calculate the net force *
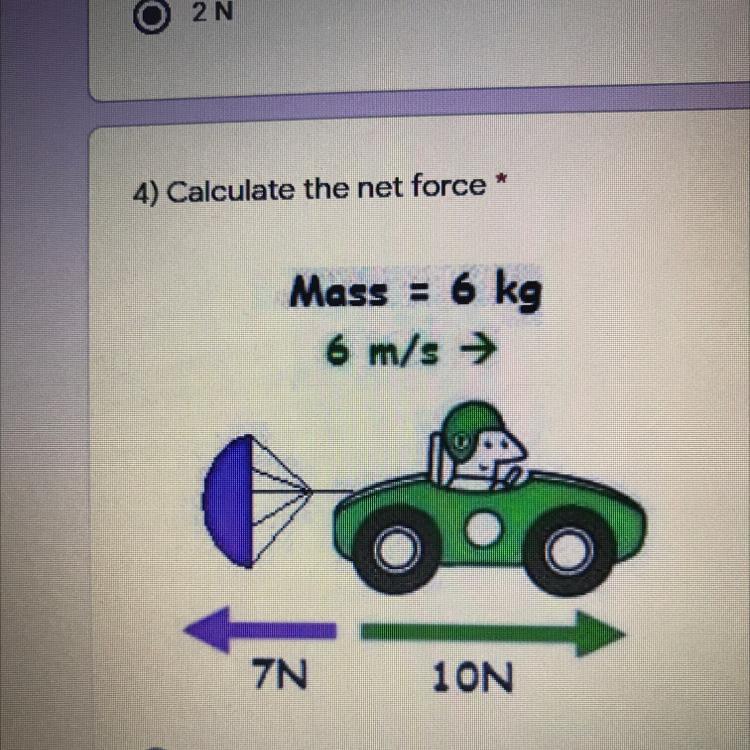
Answers
3 N
10-7= 3
the arrows are opposing each other.
Answer:
The net or resultant force depende on the direction we can easily understood the directions are opposite .Force is vector so if vectors are opposite we difference the two vectors.
10N-7N=3N
Hydrogen and oxygen combine to form water in a combustion reaction. The Gibbs energy for this reaction is negative at 773K. Is this reaction spontaneous? Explain why or why not.
Answers
The reaction is spontaneous because the Gibbs free energy for the reaction is negative at the given temperature
Gibbs free energyΔG = ΔH – TΔS
Where
ΔG is the Gibbs free energy ΔH is the enthalpy changeT is the temperature ΔS is the change in entropyNOTE
ΔG = +ve (non spontaneous) ΔG = 0 (equilibrium) ΔG = –ve (spontaneous)Considering the question given above, we were told that the Gibbs free energy for the reaction is negative at 773 K.
Thus, we can conclude that the reaction is spontaneous at the given temperature.
Learn more about Gibbs free energy:
https://brainly.com/question/9552459
#SPJ1
Given+the+following+information,+calculate+the+molecular+formula:+c+=+40.00%;+h+=+6.71%;+o+=+53.28%;+molar+mass+=+90.08+g/mol
Answers
The molecular formula of the compound is C3H6O2, indicating that there are 3 carbon atoms, 6 hydrogen atoms, and 2 oxygen atoms in one molecule.
To calculate the molecular formula, we need to determine the ratio of each element present in the compound. Given the percentages of carbon (C), hydrogen (H), and oxygen (O) in the compound as 40.00%, 6.71%, and 53.28% respectively, we can assume a 100 gram sample.
Convert the percentages to grams:
C: 40.00% of 100 g = 40.00 g
H: 6.71% of 100 g = 6.71 g
O: 53.28% of 100 g = 53.28 g
Convert the grams to moles:
C: 40.00 g / 12.01 g/mol (molar mass of carbon) = 3.33 mol
H: 6.71 g / 1.01 g/mol (molar mass of hydrogen) = 6.64 mol
O: 53.28 g / 16.00 g/mol (molar mass of oxygen) = 3.33 mol
Divide the moles by the smallest number of moles:
C: 3.33 mol / 3.33 mol = 1
H: 6.64 mol / 3.33 mol = 2
O: 3.33 mol / 3.33 mol = 1
Therefore, the molecular formula of the compound is C3H6O2.
Learn more about Molecular formula
brainly.com/question/28647690
#SPJ11
Write balanced, net ionic equations for the reactions shown below. Identify the spectator ions. (40
points possible: 8 points per problem = 4 points for net ionic equation, 4 points for spectator ions)
a. Sr(NO3)2 (aq) + H2SO4 (aq) → SrSO4 (s) + 2HNO3 (aq)
Answers
Taking into account the definition of net ionic equation, the net ionic equation is Sr²⁺(aq) + 2 SO₄⁻(aq) → SrSO₄ (s) and H⁺ and NO₃⁻ are the spectator ions.
The net ionic equation is a chemical equation for a reaction that lists only the species that participate in the reaction.
In other words, an ionic equation is a chemical equation where electrolytes in aqueous solution are written as dissociated ions. Usually this is a salt dissolved in water, where the ionic species are followed by (aq) in the equation, to indicate that they are in aqueous solution.
The balanced equation will be:
Sr(NO₃)₂ (aq) + H₂SO₄ (aq) → SrSO₄ (s) + 2 HNO₃ (aq)
where (aq) means aqueous and (s) solid.
Taking into account that:
All salts of NO₃⁻, C₂H₃O₂⁻, ClO₃⁻ and ClO₄⁻ are soluble.The total ionic equation in separated aqueous solution will be:
Sr²⁺(aq) + 2 NO₃⁻(aq) + 2 H⁺(aq) + 2 SO₄⁻(aq) → SrSO₄ (s) + 2 NO₃⁻(aq) + 2 H⁺(aq)
A spectator ion is an ion that appears both as a reactant and as a product in an ionic equation.
Spectator ions can be either cations (positively charged ions) or anions (negatively charged ions).
When writing a net ionic equation, spectator ions found in the original equation are ignored. Thus, the total ionic reaction is different from the net chemical reaction.
In this case, H⁺ and NO₃⁻ are the spectator ions because they appear unchanged in both the product and the reagent. So these ions cancel out by writing the net ionic equation and you get:
Sr²⁺(aq) + 2 SO₄⁻(aq) → SrSO₄ (s)
In summary, the net ionic equation is Sr²⁺(aq) + 2 SO₄⁻(aq) → SrSO₄ (s) and H⁺ and NO₃⁻ are the spectator ions.
Learn more:
brainly.com/question/18896765?referrer=searchResults brainly.com/question/10553963?referrer=searchResults brainly.com/question/7018960 brainly.com/question/24099019?referrer=searchResults brainly.com/question/10538922?referrer=searchResultsWhen the temperature of a rigid hollow sphere containing 685 L of helium gas is held to 62C, the pressure of the gas is 1.89 * 10 ^ 3 * kPa . How many grams of helium does the sphere contain? (Round to 3 significant digits)
Answers
mass of helium = 18.6 g. To answer this question, we can use the ideal gas law, which relates the pressure, volume, temperature, and amount of a gas.
According to 1, the ideal gas law is often written as:
PV = nRT
where P is the pressure of the gas, V is the volume of the gas, n is the number of moles of the gas, R is the ideal gas constant and T is the temperature of the gas.
We are given the values of P, V and T, and we need to find n. We can rearrange the equation to solve for n:
n = PV/RT
We also need to make sure that we use consistent units for P, V, T and R. According to 2, if we use R = 8.31 J/K/mol, then we must use P in pascals (Pa), V in cubic meters (m³), and T in kelvins (K).
We can convert the given values to these units as follows:
P = 1.89 x 10³ kPa x 10³ Pa/kPa = 1.89 x 10⁶ Pa
V = 685 L x 10⁻³ m³/L = 0.685 m³
T = 62°C + 273.15 = 335.15 K Plugging these values into the equation for n, we get:
n = (1.89 x 10⁶ Pa) x (0.685 m³) / (8.31 J/K/mol) / (335.15 K)
n = 4.64 mol
To find the mass of helium in grams, we need to multiply n by the molar mass of helium, which is 4 g/mol according to 3. Therefore,
mass of helium = 4.64 mol x 4 g/mol = 18.6 g
Rounding to three significant digits, we get:
mass of helium = 18.6 g
To know more about ideal gas law, visit:
https://brainly.com/question/28257995
#SPJ1