what quanity of energy in kilojoules is needed to vaporize 28.6 g sample of liquid benzene at its normal boiling point of 80 c
Answers
At benzene's typical boiling point of 80 c, 11.30 kilojoules will be required to vaporise a 28.6 g sample of liquid benzene.
What is the vaporization's molar heat?
The heat that one mole of a substance absorbs during the transition from a liquid to a gas is known as the molar heat of vaporisation (Hvap).
The usual boiling point of benzene (C6H6) is 80.1 °C, and the enthalpy of vaporization is 30.8 kJ/mol.
Molecular heat of vaporization
ΔH∘ = (mass/molar mass)* enthalpy of vaporization
ΔH∘ = 28.6 g / 78 g/mole * 30.84 kJ
ΔH∘ = 11.30 kJ
At room temperature, the chemical benzene is a colourless or pale yellow liquid. It smells pleasant and is quite combustible. Benzene swiftly disappears into the atmosphere. Its vapor can descend into low-lying locations since it is heavier than air.
To know more about benzene, visit
https://brainly.com/question/14525517
#SPJ4
Related Questions
Find the element that is oxidized and the one that is reduced KClO3 + 6 FeSO4 + 3 H2SO4 --> KCl + 3 Fe2(SO4)3 + 3 H2O
Answers
Answer: Fe is the element that is being oxidized (oxidation number changes from +2 to +3) while Cl is the element that is reduced (oxidation number changes from +5 to -1) in the given reaction.
Explanation:
The question requires us to find the element that is oxidized and the one reduced in the following chemical reaction:
\(KClO_3+6FeSO_4+3H_2SO_4\rightarrow KCl+3Fe_2(SO_4)_3+3H_2O\)To solve this problem, we need to determine the oxidation number of all elements in the reactants and products sides, and then identify the elements that had their oxidation number increased (oxidized species) and decreased (reduced species).
To identify the oxidation numbers, we'll need to remember a few points:
- K is an alkali metal (part of group 1 in the periodic table), and it usually assumes the oxidation number +1;
- O usually presents oxidation number -2, except in a few specific cases;
- the anion (SO4) presents total charge -2 (in this anion, S presents oxidation number +6 and O, -2);
- H usually presents oxidation number +1.
Next, let's identify the oxidation number of all elements involved in the reaction, on both sides of the chemical equation. Since all compounds are neutral (i.e., they do not present charge), the sum of all oxidation numbers must be 0:
(note that the oxidation number of each element is indicated in red, above the respective element, while the contribution of this element to the molecule charge, considering the number of atoms, is represented in blue below the element).
In the image above, we can see that Cl has its oxidation number changing from +5 in KClO3 to -1 in KCl (highlighted in purple), while Fe has its oxidation number changing from +2 in FeSO4 to +3 in Fe2(SO4)3 (highlighted in green).
Therefore, we can say that Fe is the element that is being oxidized while Cl is the element that is reduced in the given reaction.

What does NIBIN stand for? (Forensic Science)
Answers
Answer:National Integrated Ballistic Information Network
Explanation:
Answer:
National Integrated Ballistic Information Network
Explanation:
gradpoint
Which condition would most likely require the national weather service to issue a severe thunderstorm warning?(1 point) a front moving out of an area a front moving out of an area an air mass developing over a large body of water an air mass developing over a large body of water a large amount of moisture in the air condensing to form clouds a large amount of moisture in the air condensing to form clouds a meeting of two air masses with major differences in temperature and moisture
Answers
The condition that would most likely require the National Weather Service to issue a severe thunderstorm warning is "a meeting of two air masses with major differences in temperature and moisture."
Severe thunderstorms often form when there is a collision between two air masses with contrasting properties. This collision can create an unstable atmosphere and trigger the development of severe weather conditions, including thunderstorms.
When two air masses with significant differences in temperature and moisture interact, it creates a strong contrast in atmospheric conditions. The warm, moist air from one air mass clashes with the cooler, drier air from the other air mass. This clash sets the stage for intense convection and the potential for severe thunderstorm formation.
The collision of these air masses results in the rapid uplift of warm, moist air, leading to the formation of towering cumulonimbus clouds. These clouds are capable of producing severe weather phenomena, such as heavy rainfall, strong winds, hail, and even tornadoes.
Given the potential hazards associated with severe thunderstorms, including the risk of property damage and threats to human safety, it is crucial for the National Weather Service to issue timely warnings to alert the public and help them take necessary precautions.
By closely monitoring the conditions of air masses and identifying areas where major differences in temperature and moisture exist, meteorologists can anticipate the potential for severe thunderstorm development. This allows them to issue appropriate warnings and advisories to keep the public informed and safe during severe weather events.
Learn more about National Weather Service here:
https://brainly.com/question/30749824
#SPJ11
For a given recipe, 14 cups of flour are mixed with 21 cups of sugar. How many cups of flour should be used if 36 cups of sugar are used?.
Answers
Please answer the following question using the data below: H2O vapor content: 13 grams H2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10 ∘
C 52 grams at 30 ∘
C What is the dew point for the conditions listed above? LCL 3π5 25C Relative Humidity =100%
Answers
Given data:H2O vapor content: 13 gramsH2O vapor capacity: 52 grams at 25 degrees Celsius 13 grams at 10∘C52 grams at 30∘CFormula used to find the dew point:$$\dfrac{13}{52}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$\frac{1}{4}=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$
Where A is the constantDew Point:It is the temperature at which air becomes saturated with water vapor when the temperature drops to a point where dew, frost or ice forms. To solve this question, substitute the given data into the formula.$$13/52=\dfrac{(A*3\pi)/(ln100)}{(17.27-A)}$$$$13(17.27-A)=3\pi A(ln100)$$By simplifying the above expression, we get$$A^2-17.27A+64.78=0$$Using the quadratic formula, we get$$A=9.9,7.4$$
The dew point is 7.4 since it is less than 10°C.More than 100:The term "More than 100" has not been used in the question provided.
To know more about temperature visit:
https://brainly.com/question/7510619
#SPJ11
What is the voltage across 200 ohm circuit element that draws a current of 4 A
Answers
The voltage across the 200 ohm circuit element, that draws a current of 4 A, is equal to 800 volts.
To find the voltage across a circuit element, take Ohm's Law, according to which the resistance (R) of a resistor is equal to the product of the current (I) that flows through it and the voltage (V) across it.
Use the values:
Current (I) = 4 A
Resistance (R) = 200 Ω
Using the formula of Ohm's Law:
V = I × R
V = 4 A × 200 Ω
V = 800 V
Voltage is 800 V.
Thus, the voltage across the 200 ohm circuit element, that draws a current of 4 A, is 800 volts.
Learn more about voltage, here:
https://brainly.com/question/23172991
#SPJ1
Name a sensory organ, and briefly explain its function.
Answers
Answer:
Eyes are sensory organs because sight is one of the major senses. Eyes process light, allowing us to differentiate colors and shapes to create an image of the surrounding environment.
Explanation:
Edmentum Sample Answer
HELP PLEASE 100 points!!!

Answers
Answer:
by the looks of it the answer you have is correct
Explanation:
Answer:
The answer B
.
Explanation:
We assumed that all the SCN^- ion was converted to FeSCN^2+ ion in Part 1 because of the great excess (approximately 1000x) of Fe^3+ ion. However, since the equilibrium shown Kf = [FeSCN^2+]/[Fe^3+][SCN^-] takes place, a trace amount of SCN^- ion must also be present.
(a) Use the Kf mean ( 312.56) to calculate the SCN^- ion concentration in solution S3 (8.0e-05M).
(b) Based on your answer in part a, was the assumption made in Part 1 valid? What percentage of SCN^- ion was converted to the FeSCN^2+ ion? Hint: For the assumption to be valid, more than 95% of the SCN^- ion should be converted to FeSCN^2+ ion.
Answers
This experiment explores the equilibrium created by the reaction between the thiocyanate (SCN-) and iron (III), Fe3+, ions.Because the equilibrium concentrations of the reactants and products remain constant.
FeSCN2+, complex ions (aq): Colorless. Colorless. Orange. Fe3+.Use the value k = 5.00 103 to calculate the concentration (M) of FeSCN2+ for each solution after recording the absorbance value. This is done by using the equation A = k M. Use a glass stirring stick to completely combine each solution before letting them sit for at least five minutes to establish equilibrium. Beer's Law and spectroscopy are used to determine the equilibrium concentration of [FeSCN2+] to be 1.50 x 10-4 M.
To know more about thiocyanate, click here:
https://brainly.com/question/29072122
#SPJ4
What is the final temperature of a sample of magnesium (c = 0.24 cal/g°C), if 1835 cal is
added to 612 g of magnesium at an initial temperature of 19.3°C?
Answers
Answer:31.8 °C
Explanation:
Delta T=?
C= .24 cal/g°C
M=612g
Q=1835
Delta T= 1835 cal/(.24cal/g°C)(612g)=12.50°C
Intial temp= 19.3°C
Add intial temp to new temp
12.50+19.3=31.8°C
a a reaction orrcurs in a calorimeter that contains 2300g of water at 30c. the reaction releases 9.66 *10^3 j of heat. if the specific heat capacity of water is 4.184 j*g*c what is the final temperature of the water
Answers
The final temperature of the water is 33.02°C.
Here's the solution:
Initial temperature of water (T1) = 30°C
Mass of water (m) = 2300g
Specific heat capacity of water (c) = 4.184 J/g°C
Heat released by the reaction (q) = 9.66 * 10^3 J
Final temperature of water (T2) = (T1 + q/mc)
= (30°C + 9.66 * 10^3 J / 2300g * 4.184 J/g°C)
= 33.02°C
The heat released by the reaction is absorbed by the water, causing the temperature of the water to increase.
The final temperature of the water is calculated by adding the heat released by the reaction to the initial temperature of the water and dividing by the mass of the water and the specific heat capacity of water.
Thus, the final temperature of the water is 33.02°C.
To learn more about specific heat capacity :
https://brainly.com/question/29792498
#SPJ11
what kind of intermolecular forces act between a water molecule and a hydrogen peroxide h2o2 molecule?
Answers
The main intermolecular forces that act between a water molecule and a hydrogen peroxide (H2O2) molecule are hydrogen bonding and dipole-dipole interactions.
Hydrogen bonding occurs between the hydrogen atom in the water molecule and the oxygen atom in the H2O2 molecule. This is because both molecules have polar covalent bonds, which result in partial charges on their atoms.
Hydrogen bonding is a type of dipole-dipole interaction, which occurs between two molecules with permanent dipoles. The oxygen atom in the water molecule is partially negative, while the hydrogen atoms are partially positive, creating a dipole.
The oxygen atoms in the H2O2 molecule are also partially negative, resulting in another dipole. These dipoles interact, leading to dipole-dipole interactions. These intermolecular forces help to hold the water and H2O2 molecules together, enabling them to mix and interact with each other.
For more questions like Hydrogen bonding click the link below:
https://brainly.com/question/13677258
#SPJ4
In a container with volume of 25.0 L, there are 40 g of CH4 gas. If the number of gas is reduced to 15.0 L, what is the new amount inmole?
Answers
Answer
1.50 mol
Explanation
Given:
Initial volume, V₁ = 25.0 L
Mass of CH4 gas in 25.0 L container = 40 g
Final volume, V₂ = 15.0 L
From the Periodic Table; molar mass of CH4 = 16.04 g/mol
What to find:
The new amount in mole.
Step-by-step solution:
According to Avogadro’s law: For a confined gas, the volume (V) and number of moles (n) are directly proportional if the pressure and temperature both remain constant. That is:
\(\frac{V_1}{n_1}=\frac{V_2}{n_2}\)n₁ = Mass/Molar mass = (40.0g/16.04 g/mol) = 2.493765586 mol
n₂ is the new amount in mole and can be calculated as follows:
\(\begin{gathered} \frac{25.0\text{ L}}{2.493765586\text{ mol}}=\frac{15.0\text{ L}}{n_2} \\ \text{Cross multiply} \\ n_2\times25.0\text{ L }=15.0\text{ L }\times2.493765586\text{ mol} \\ \text{Divide both sides by 25.0 L} \\ \frac{n_2\times25.0\text{ L}}{25.0\text{ L}}=\frac{15.0\text{ L }\times2.493765586\text{ mol}}{25.0\text{ L}} \\ n_2=1.496259352\text{ mol} \\ To\text{ 3 significant digits} \\ n_2=1.50\text{ mol} \end{gathered}\)The new amount in moles is 1.50 moles
How have animals developed physiological adaptations to survive in colder climates?
Answers
35.0 mL of a 0.250 M solution of KOH is titrated with 0.150 M HCl. After 35.0 mL of the HCl has been added, the resultant solution is: Question 2 options: Acidic and before the equivalence point Basic and after the equivalence point Neutral and at the equivalence point Basic and before the equivalence point Acidic and after the equivalence point
Answers
Since only 35.0 mL of HCl is added, the resultant solution is still basic and before the equivalence point. Therefore, the correct option is Basic and before the equivalence point.
The given data is:35.0 mL of a 0.250 M solution of KOH is titrated with 0.150 M HCl. After 35.0 mL of the HCl has been added, the resultant solution is to be determined.
The balanced chemical equation is: KOH + HCl → KCl + H2O
The number of moles of KOH can be calculated as:
Number of moles = Molarity × Volume
Number of moles of KOH = 0.250 M × (35.0 / 1000) L = 0.00875 moles
Similarly, the number of moles of HCl can be calculated as:
Number of moles of HCl = 0.150 M × (35.0 / 1000) L = 0.00525 moles
Now, the limiting reagent in this reaction is HCl because it is present in less number of moles. Therefore, HCl is completely consumed and KOH is present in excess.
The balanced chemical equation shows that 1 mole of HCl neutralizes 1 mole of KOH. Therefore, the number of moles of HCl required to neutralize 0.00875 moles of KOH is also 0.00875 moles.
Thus, the volume of HCl required to neutralize KOH is:
Number of moles = Molarity × Volume
Volume of HCl required = 0.00875 moles / 0.150 M
= 0.0583 L or 58.3 mL
Since only 35.0 mL of HCl is added, the resultant solution is still basic and before the equivalence point. Therefore, the correct option is Basic and before the equivalence point.
To learn more about equation visit;
https://brainly.com/question/29538993
#SPJ11
please i just want the answer for the boxes if you know the right answer please tell me please
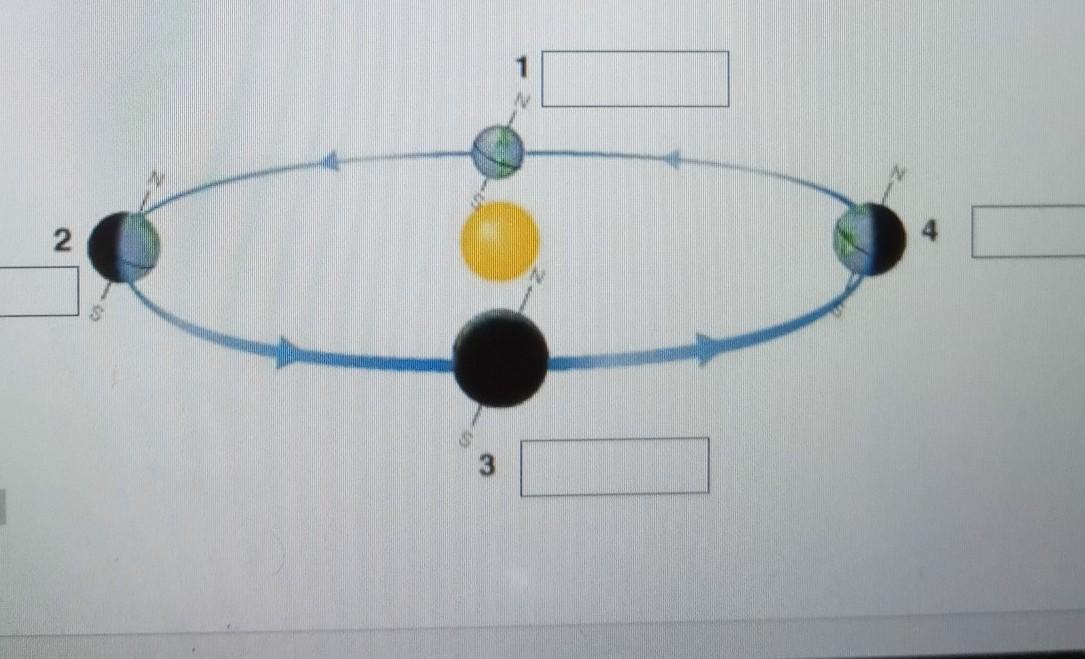
Answers
Answer:
1. summer 2 spring 3 winter 4 fall/autumn
Infrared and microwaves are two types of electromagnetic radiation.
(a) State one example of the use of each type of radiation for communication.
Answers
Infrared and microwaves are two sorts of electromagnetic radiation that are commonly utilized for communication purposes.
What are examples of electromagnetic radiation?One case of the utilize of infrared radiation for communication is in inaccessible controls for electronic gadgets. Infrared signals are utilized to transmit commands from the farther control to the gadget, such as changing the channel on a tv or altering the volume on a sound framework.
One case of the utilize of microwaves for communication is in cellular phone systems. Microwaves are utilized to transmit data between cell phone towers and versatile gadgets. The recurrence of the microwaves utilized in cell phone systems is regularly within the run of 800 MHz to 2.5 GHz.
Learn more about electromagnetic radiation from
https://brainly.com/question/29696752
#SPJ1
What is the Hall coefficient (RH) in Ccc if the acceptor doping is 4.18∗10∧15/cc, and the donor doping is 9.40∗10∧15/cc ? Three significant figures and exponential notation 1.23e−4
Answers
The Hall coefficient (RH) in this case is approximately -3.01 * 10^-6 C^-1 cc (rounded to three significant figures in exponential notation).
The Hall coefficient (RH) is a parameter used to describe the behavior of charge carriers in a material when subjected to a magnetic field. It is given by the equation RH = 1/(e * p) where e is the elementary charge and p is the total charge carrier density. In this case, we are given the acceptor doping concentration (Na) and the donor doping concentration (Nd) in units of /cc.
To calculate the Hall coefficient, we need to determine the total charge carrier density (p). The total charge carrier density can be calculated as the difference between the acceptor doping concentration and the donor doping concentration: p = Na - Nd.
Given the acceptor doping concentration Na = 4.18 * 10^15/cc and the donor doping concentration Nd = 9.40 * 10^15/cc, we can substitute these values into the equation to find p:
p = Na - Nd
= (4.18 * 10^15/cc) - (9.40 * 10^15/cc)
= -5.22 * 10^15/cc
Now, we can substitute the value of p into the Hall coefficient equation:
RH = 1/(e * p)
= 1/(1.60 * 10^-19 C * (-5.22 * 10^15/cc))
= -3.01 * 10^-6 C^-1 cc
To know more about magnetic field visit:
https://brainly.com/question/19542022
#SPJ11
Which object is at the center of the solar system in the heliocentric model?
A. The asteroid belt
B. The Sun
C. Earth
D. The Moon
Answers
In the heliocentric model, the object at the center of the solar system is the Sun. Therefore, the answer is B.
What is the heliocentric model?
The heliocentric model is a theory that places the Sun at the center of the solar system, with all the planets orbiting around it. This model was first proposed by the ancient Greek astronomer Aristarchus of Samos in the 3rd century BCE, but it was not widely accepted until the 16th century when the Polish astronomer Nicolaus Copernicus presented a detailed mathematical description of the heliocentric model.
The heliocentric model provided a more accurate description of the solar system, and it was later confirmed by the observations and calculations of other astronomers such as Johannes Kepler and Galileo Galilei. The heliocentric model is now widely accepted, and it forms the basis of modern astronomy.
To know more about the solar system, visit:
https://brainly.com/question/29608606
#SPJ1
The complete question is: The Sun is at the center of the solar system in the heliocentric model.
The speed of light is 300,000,000 m/s. What is the frequency of microwaves with a wavelength of 0.01 meter?
Answers
Answer:
30,000,000,000 Hz
Explanation:
I searched it up and got 3 x 10^10 and thats 30000000000
Match the statements for the three principles for Newton's Law of Gravitation.
1 .
Gravitational force
attracts every other object.
2 .
Every object
depends on the quantity of mass.
3 .
Distance between objects
factor that also affects the force of gravity.
please answer quick grrrr betch as neggas
Answers
According to the principles of gravitation by Newton, every objects attracts every other objects. The gravitational force depends on the quantity of mass and the distance between objects factor that also affects the force of gravity.
What is gravitational force?Gravitational force is a kind of force by which an object attracts other objects into its centre of mass. This force of attraction depends on the mass of both objects.
Gravity is directly proportional to the mass of the object and inversely proportional to the distance between the objects. Thus force of gravity increases with mass and decreases with distance.
The sentences can be completed as:every objects attracts every other objects. The gravitational force depends on the quantity of mass and the distance between objects factor that also affects the force of gravity.
To find more on gravity, refer here:
https://brainly.com/question/4014727
#SPJ1
Answer:Match the statements for the three principles for Newton's Law of Gravitation.
1 .
Gravitational force
attracts every other object.
2 .
Every object
depends on the quantity of mass.
3 .
Distance between objects
factor that also affects the force of gravity.
please answer quick grrrr betch as neggas
Explanation:
What are the cons of using a cup made of steel?
Answers
Answer:
1. Sometimes there’s a metallic taste to the water
2. The water becomes hot if left in your car or outdoors in hot weather
3. Bottle can dent if dropped
4. Paint sometimes peels off exterior of metal bottles
5. Metal water bottles lined with a resin lining also leach BPA. Do not get a bottle with what appears to be a “colored” lining of any kind.
Explanation:
Answer:
Steel feels cold, can dent, and is heavy. Sometimes it has a strange taste. Steel can also stain with use.
Explanation:
Hope this helps! :)
B-
Why many nuclei like U234, U236 and
U238 undergo fission only by fast neutrons?
Answers
The nucleus more easily, increasing the probability of causing fission many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission can be caused by slow as well as fast neutrons. It is the energy of the neutron which determines its effectiveness in causing fission. Fast neutrons are more effective in causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fission is a nuclear reaction process in which the nucleus of an atom is split into two smaller nuclei with the release of a large amount of energy and two or three neutrons. Uranium-235 (U-235) and Plutonium-239 (Pu-239) are the most commonly used fissile materials, but other materials like U-234, U-236, and U-238 can also undergo fission. When a neutron is absorbed by the nucleus of a fissile material like U-235 or Pu-239, it becomes unstable and splits into two smaller nuclei with the release of a large amount of energy.
The fission process also releases two or three neutrons, which can cause further fission of other nuclei, leading to a chain reaction. The chain reaction can be controlled by using a neutron moderator, which slows down the fast neutrons, making them more effective in causing fission. The efficiency of the fission reaction depends on the energy of the neutron.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy. This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Fast neutrons are more effective in causing fission than slow neutrons, which have less energy.
This is because fast neutrons can penetrate the nucleus more easily, increasing the probability of causing fission. Hence, many nuclei like U234, U236, and U238 undergo fission only by fast neutrons.
Learn more about nucleus with the given link,
https://brainly.com/question/13061744
#SPJ11
What is the molarity of 0.25 moles of KBr dissolved into 250 ml of solution?
Answers
Answer: 1.0 M
Explanation:
250 mL = 0.25 L
molarity = (moles of solute)/(liters of solution) = 0.25/0.25 = 1.0 M
3. If you had 3.4 moles of NaOH, how many moles of H2SO4 would it take to neutralize the
solution?
Helppp
Answers
Answer:Suppose that H2SO4 was used in the reaction instead of HCl. How many moles of NaOH would neutralize 1 mole of H2SO4?
the answer is 2
Explanation:
this is the number of protons and neutrons i the nucleus of any atom
Answers
Answer:
The mass number is defined as the total number of protons and neutrons in an atom. Consider Table below which shows data from the first six elements of the periodic table. Consider the element helium. Its atomic number is 2, so it has two protons in its nucleus.
Explanation:
what can vanilla extract be used for around the house?
Answers
The vanilla extract can be used in the house to;
Deodorising the Microwave.Freshen Up the Fridge.What is vanilla extract ?Vanilla beans are steeped in ethyl alcohol and water to produce vanilla extract, an aromatic, amber-colored liquid. pure vanilla extract is gotten through macerating and percolating vanilla pods in an ethanol and water mixture, vanilla extract is created.
In many Western sweets, particularly baked goods like cakes, cookies, brownies, and cupcakes, as well as custards, ice creams, and puddings, it is seen as a necessary component.
Learn more about vanilla extract at
https://brainly.com/question/19017839
#SPJ1
when 50 ml of 0.10 m naf is added to 50 ml of 0.10 m hf, relative to the ph of the 0.10 m hfsolution the ph of the resulting solution willa. remain the sameb. become 7c. increased. decrease
Answers
When 50 ml of 0.10 m NaF is added to 50 ml of 0.10 m HF, relative to the pH of the 0.10 m HF solution the pH of the resulting solution will increased. the option c increased is correct.
The pH is the measurement for the solution that it is acidic or the basic or it is neutral. the pH scale ranges from 0 to 14 in the scale. the pH value in the 7 is for the neutral solution. that means it is neither basic nor acidic. the pH value greater than 7 will be the basic solution. the pH value in the pH scale less than 7 is for the acidic solution.
Thus, the pH will increases as compared to the value of the 0.10 M HF. the option c is correct.
To learn more about pH here
https://brainly.com/question/29570541
#SPJ4
Which of these metals must release the most heat to expenence a given decrease in temperature per gram of metal? Aluminum, Lead, and Iron.
Answers
Aluminium is the metal which must release the most heat to experience a given decrease in temperature per gram of metal.
What is Thermal conductivity?This is as a result of free electrons and is the ability of a substance to conduct heat.
Aluminium has the highest thermal conductivity in this case which is why it will release the most heat to experience a given decrease in temperature per gram of it.
Read more about Thermal conductivity here https://brainly.com/question/11213835
#SPJ1
2) 2KClO3 --> 2KCl + 3O2
a) How many moles of O2 are produced from 19 moles of KClO3?
b) How many kilograms of KClO3 would decompose to form 62 moles of KCl?
c) How many grams of O2 are required to react with 39 grams of KCl?
show work
Answers
\(2 \text{ KClO}_3 \to 2 \text{ KCl}+3\text{ O}_2\)
a)
\(2 \text{ mols of KClO}_3 \equiv 3 \text{ mols of O}_2\)
\(19 \text{ mols of KClO}_3 \equiv 3\cdot 9,5 \text{ mols of O}_2\)
\(\boxed{19 \text{ mols of KClO}_3 \equiv 28,5 \text{ mols of O}_2}\)
b)
\(2 \text{ mols of KClO}_3 \equiv 2 \text{ mols of KCl}\)
\(62 \text{ mol of KClO}_3 \equiv 62 \text{ mol of KCl}\)
Using the atomic mass given in the periodic table:
\(62\cdot(39+35,5+16\cdot3) \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(62\cdot122,5 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(7595 \text{ g of KClO}_3 \equiv 62 \text{ mol of KCl}\)
\(\boxed{7,595 \text{ kg of KClO}_3 \equiv 62 \text{ mol of KCl}}\)
c)
\(2 \text{ KCl}+3\text{ O}_2\to 2 \text{ KClO}_3\)
\(3 \text{ mols of O}_2 \equiv 2 \text{ mols of KCl}\)
Using the atomic mass given in the periodic table:
\(3\cdot(2\cdot 16) \text{ g of O}_2 \equiv 2\cdot(39+35,5) \text{ g of KCl}\)
\(96\text{ g of O}_2 \equiv 149\text{ g of KCl}\)
\(\dfrac{39}{149}\cdot 96\text{ g of O}_2 \equiv \dfrac{39}{149}\cdot 149\text{ g of KCl}\)
\(\boxed{25,13\text{ g of O}_2 \equiv 39\text{ g of KCl}}\)
This result is an aproximation.