What type of orbitals best describe the bond between the adjacent oxygens in peroxyacetic acid?
Answers
sp³-sp³ type of bonds best describes the bond between the adjacent oxygen.
What are orbitals?An atomic orbital is a function that describes the position and wave-like activity of an electron in an atom in terms of both atomic theory and quantum mechanics. This function may be used to determine the likelihood of discovering any atom's electron in any certain area surrounding the nucleus.
The electron cloud model is used by chemists to map out the electrons' atomic orbitals; these probability maps are not all spherical. The periodic table's trends can be predicted in part by their forms.
to learn more about orbitals go to -
https://brainly.com/question/26000020
#SPJ4
Related Questions
Which set of elements is in the order of decreasing/ increasing malleability? (Questions: 7 & 8)

Answers
Answer:the first answer is correct and 8 is O, Ge, Mn
Explanation:
Please answer it’s due today. I will give brainliest
Answers
Answer: It depends
Explanation: If you’re using an electric kettle then yes, it’s true!
What is the chemical formula of water? Is there a difference in the chemical formula of water when it is found naturally in rain or the ocean as opposed to scientists in a lab synthesizing water?
Answers
The chemical formula of water is H2O, which indicates that each water molecule consists of two hydrogen atoms and one oxygen atom.
The chemical formula of water is the same whether it is found naturally in rain or the ocean or synthesized in a lab. In natural sources of water, such as rain and the ocean, water is created through a variety of natural processes involving the water cycle, which includes evaporation, precipitation, and condensation.
However, when water is synthesized in a lab, it is typically done by combining hydrogen gas and oxygen gas in the presence of a catalyst.
To learn more about chemical formula refer to:
brainly.com/question/29031056
#SPJ4
Chalk is a silicate carbonate evaporite sandstone QUESTION 33 a photosyntehtic creature with a silica shell can be a O coccolithophorid foraminifer diatom radiolarian QUESTION 34 recrystallization of chalk at the ocean bottom (not in metamorphic conditions) can give us O micrite chert marble quartzite
Answers
Diatoms are single-celled algae that have a silica (silicate) shell called a frustule.
Diatoms are photosynthetic organisms and are known for their intricate and diverse shapes. Diatoms are commonly found in freshwater and marine environments and play a significant role in the global carbon cycle.
Micrite is a fine-grained carbonate sedimentary rock composed of tiny carbonate particles. It forms through the precipitation and accumulation of carbonate minerals, such as calcite or aragonite, in marine environments. In the case of chalk, which is primarily composed of microscopic fragments of calcium carbonate from marine organisms, recrystallization can occur at the ocean bottom under specific conditions, leading to the formation of micrites.
Therefore, it's important to note that chert, marble, and quartzite are not the typical products of recrystallization of chalk at the ocean bottom.
For more details regarding diatoms, visit:
https://brainly.com/question/11446176
#SPJ4
6
What is the density of a substance that has a mass of 2.0 g, and when placed in a graduated cylinder
the volume changed from 70 mL to 75 mL?
A 2.5 g/mL
B 7.0 g/mL
C 10. g/mL
D 0.40 g/mL
Answers
The density of the substance having a mass of 2.0 g is 0.4 g/mL (Option D)
How do I determine the density of the substance?First, we shall obtain the volume of the substance. This can be obtained as follow:
Volume of water = 70 mL Volume of water + substance = 75 mL Volume of substance =?Volume of substance = (Volume of water + substance) - (Volume of water)
Volume of substance = 75 - 70
Volume of substance = 5 mL
Finally, we shall determine the density of the substance. This is illustrated below:
Mass of substance = 2.0 gVolume of substance = 5 mLDensity of substance = ?Density = mass / volume
Density of substance = 2 / 5
Density of substance = 0.4 g/mL
Thus, the density is 0.4 g/mL (Option D)
Learn more about density:
https://brainly.com/question/952755
#SPJ1
Identify the combustion reaction

Answers
Answer:
C₄H₁₂ + 7O₂ --> 4CO₂ + 6H₂O
Explanation:
Organic molecules react with O2 to create water and CO2 in combustion processes. C4H12 is an organic molecule that combines with O2 to create water and CO2 as shown in the reactions.
As a result, this is the sole reaction that obeys the general combustion equation.
8. GIVEN 0.289 mole of Cag(PO4)3F? find the number of grams.
Answers
If the metal from problem 4 was initially at room temperature (22 0 C), what would the final temperature of the metal be? You know that you add 120 joules of energy to the metal. What change in temperature would you observe Q = is energy as Heat, 120 Joulesm = mass in grams, 5.0 gramsc = is the specific heat capacity, 0.385 J/g°CΔT = the change in temperature, calculated as Final Temperature - Initial T120 = 5 * 0.385 * T120 = 1.925TT = 62°C of change in temperature
Answers
The question is mostly solved. The definition of heat is used for this problem which tells us:
\(Q=mCp\Delta T\)Where,
Q is the heat added to the system, 120 J
m is the mass of the metal, 5.0 g
Cp is the specific heat of the metal, 0.385J/g°C
dT is the change of temperature:
\(\Delta T=T_2-T_1\)T2 is the final temperature, unknown
T1 is the initial temperature, 22°C
We clear the final temperature from the equation:
\(\begin{gathered} Q=mCp(T_2-T_1) \\ Q=mCpT_2-mCpT_1 \\ T_2=\frac{Q+mCpT_1}{mCp} \end{gathered}\)Now, we replace the known data:
\(T_2=\frac{120J+5.0g\times0.385\frac{J}{g\degree C}\times22\degree C}{5.0g\times0.385\frac{J}{g\degree C}}\)\(\begin{gathered} T_2=\frac{120+5.0\times0.385\times22}{5.0\times0.385}\degree C \\ T_2=84\degree C \end{gathered}\)Answer:
The final temperature of the metal will be 84°C
The change in the temperature will be 84°C-22°C=62°C
DEFINE HEAT please help!

Answers
Answer:
A
Explanation:
heat is how hot something is
na mainit na mainit
Which is true for a substance that absorbs energy?
A The energy increases the molecular motion and the kinetic energy of the substance.
B The energy decreases the molecular motion but increases the kinetic energy of the substance.
C The energy decreases the molecular motion and the kinetic energy of the substance.
D The energy increases the molecular motion but decreases the kinetic energy of the substance.
Reset
Next
Answers
Answer:
A. The energy increases the molecular motion and the kinetic energy of the substance.
Explanation:
When a substance absorbs energy, the molecules will move faster because there will be more vibrations and collisions.
As a result of more molecular motion, the kinetic energy will increase because there is more movement.
Match the element with its description. Match Term Definition Sodium A) Has properties of both metals and nonmetals Silicon B) Highly reactive gas Bromine C) Malleable, soft, and shiny Argon D) Nonreactive gas
Answers
Answer:
Sodium - malleable, soft, and shiny
Silicon - has properties of both metals and nonmetals
Bromine - highly reactive gas
Argon - non-reactive gas
Explanation:
Sodium is an alkaline metal. Just like other alkaline metals, it's malleable, soft, and shiny.
Silicon is a metalloid. Metalloids are elements that have properties of both metals and nonmetals.
Bromine a highly reactive chemical element. It is a fuming red-brown liquid at room temperature that evaporates to form a similarly coloured gas.
Argon is a noble gas. Just like other noble gases, it's non-reactive.
Given that Delta. G for the reaction below is –957. 9 kJ, what is Delta. Gf of H2O? 4NH3(g) 5O2(g) Right arrow. 4NO(g) 6H2O(g) Delta. Gf,NH3 = -16. 66 kJ/mol Delta. Gf,NO = 86. 71 kJ/mol –228. 6 kJ/mol –206. 4 kJ/mol 46. 7 kJ/mol 90. 7 kJ/mol.
Answers
ΔG for the formation of H₂O is -228.6 kJ.
How we calculate Gibb's free energy of the reaction?Gibb's free energy of the reaction is calculated as:
ΔG = G for product - G for reactant
Given chemical reaction is:
4NH₃(g) + 5O₂(g) → 4NO(g) + 6H₂O(g)
In the question, given that:
ΔG for the reaction = -957. 9 kJ
ΔGf of NH₃ = -16. 66 kJ/mol
ΔGf of NO = 86. 71 kJ/mol
Equation for ΔG will be written as:
ΔG = (ΔGf of NO + ΔGf of H₂O) - (ΔGf of NH₃+ ΔGf of O₂)
ΔGf of O₂ = 0
-957. 9 = (4×86. 71 + 6×ΔGf of H₂O) - (4×-16. 66 + 5×ΔGf of O₂)
-957. 9 = 346.84 + 6ΔGf of H₂O + 66.64
ΔGf of H₂O = (-957. 9 - 346.84 - 66.64) / 6
ΔGf of H₂O = -228.56 kJ ≅ -228.6 kJ
Hence, option (1) is correct i.e. -228.6 kJ is the ΔGf of H₂O.
To know more about Gibb's free energy, visit the below link:
https://brainly.com/question/14415025
Guys I need ur help I might as well just ask ur smarties for help ahah n yup I’ll be brainlisting;)
1) What have been the wider effect on the community of nuclear accidents? (Chernobyl)
Answers
Answer:
Hey sis
will you be my sis??
please give reply
I will be waiting
and fol.low.me
Two students were discussing the Mono Lake ecosystem. The first student said, "For a 20-kilogram (kg) coyote to survive, it takes around 200 kg of Wilson's phalaropes, 400 kg of brine shrimp, and 800 kg of algae!" The second student said, "No, I think it takes even more than that." 1. Which student is correct? What is your reasoning?
Answers
Answer:
The second student is right.
Explanation:
The coyote feed on not only phalaropes but many other organisms present in the environment for its survival. There are many other organisms present in the ecosystem such as mice, squirrel, cactus fruit etc. The coyote feeds on phalaropes, the phalaropes feeds on brine shrimp and the brine shrimp feeds on algae for its survival so in this way the ecosystem moves in the forward direction. The coyote feeds on phalaropes so the energy that is present in phalaropes transferred into coyote which only 10 % while the remaining is released in the atmosphere in the form of heat energy.
chemicals (and humans) are all about what
Answers
Answer:
Atoms, and chemical make up
Explanation:
Our bodies and any other terrestrial body are formed with atoms, those same atoms from a chemical make-up that creates items, such as water with 2 hydrogen atoms and 1 oxygen atom is formed. In essence, we all are about atoms and chemical makeup is the instruction on how to build us
what is a atomic bond
Answers
2.Electrons can be shared between neighbouring atoms.
3.Electrons can be shared with all atoms in a material.
Answer: A bond formed between atoms due to sharing or complete transfer of electrons is termed as "atomic bond". These are the intramolecular forces that hold atoms together in molecules.
An object has a mass of 183.5 g and a density of 14.8 g/cm³. Determine the volume of the objectin cm³.
Answers
First, let's remember the formula to calculate an object's density:
\(\begin{gathered} \rho=\text{ }\frac{m}{V} \\ \\ Being\text{ }\rho\text{ the density, m the mass, and V the volume.} \end{gathered}\)Then, we analyze what we have:
\(\begin{gathered} m\text{ = 183.5 g} \\ \rho=\text{ 14.8 g/cm}^3 \end{gathered}\)We need to determine the volume, so we transform our formula like this:
\(V=\text{ }\frac{m}{\rho}\)We replace our data:
\(V=\text{ }\frac{183.5\text{ g}}{14.8\text{ g/cm}^3}=\text{ 12.399 cm}^3\approx\text{ 12.4 cm}^3\)Then, the answer is that the volume equals 12.4 cm^3.
27.2Pb(NO3)2 --> 2Pb0 + 4NO2 + O2
O A. Synthesis
B. Single displacement
C. Decomposition
D. Combustion
Answers
Consider the balanced equation. PCl3 + 3H2O Right arrow. H3PO3 + 3HCl What is the percent yield of HCl if 42.0 g of HCl are produced from the reaction of 62.0 g of PCl3? Use Percent yield equals StartFraction actual yield over theoretical yield EndFraction times 100..
Answers
Answer:
Percient yield of reaction: 85 %
Explanation:
The reaction is:
PCl₃ + 3H₂O → H₃PO₃ + 3HCl
We only have data from the chloride, so we assume water is in excess. We convert the mass to moles:
62 g . 1mol / 137.32 g = 0.452 mol
If ratio is 1:3, from 0.452 mol we would produce three times as big, that amount. (0.452 . 3) = 1.36 moles of HCl.
We convert the amount to mass.
1.36 mol . 36.45g / 1mol = 49.4 g
This value of produced HCl is the theoretical yield. To determine the percent yield we make:
(Yield produced / Theoretical yield) . 100
(42 g / 49.4g) . 100 = 85%
Answer:
Yes it is 85.0%
Explanation:
What are these smooth, distinct layers most directly evidence of ?
Answers
Answer: The layers of the rocks in one region of the parks are smooth and distinct, which are evidence of many, many years of deposition. The layers on the rocks are because of different deposition of sediments. Different sediments deposited over the rocks through wind, water and ice over the ages
Explanation:
Answer: The layers of the rocks in one region of the parks are smooth and distinct, which are evidence of many, many years of deposition. The layers on the rocks are because of different deposition of sediments. Different sediments deposited over the rocks through wind, water and ice over the ages
Hope we helped alot! :)
Use the Text Submission box to answer the following question.
What are the major organic products are formed when the following compounds react with methylmagnesium bromide (CH₃MgBr), followed by the addition of dilute acid? a. propanal b. propanone
Answers
When propanal reacts with methylmagnesium bromide (CH₃MgBr) followed by the addition of dilute acid, the major organic product formed is 3-hydroxypropanal.
On the other hand, when propanone reacts with methylmagnesium bromide (CH₃MgBr) followed by the addition of dilute acid, the major organic product formed is 3-hydroxy-2-methylpropanal.
A dilute acid is a solution that contains a relatively small amount of acid dissolved in a solvent, usually water. Dilute acids are commonly used in various chemical and industrial processes, as well as in the laboratory.
In a dilute acid solution, the concentration of acid is low enough that it is not considered to be a concentrated or strong acid. The strength of an acid refers to its ability to donate protons (H+) to a solution, and is related to the concentration of the acid in the solution. Dilute acids typically have a lower pH value and are less reactive than concentrated acids.
Common examples of dilute acids include dilute hydrochloric acid (HCl), dilute sulfuric acid (H2SO4), and dilute nitric acid (HNO3). These acids are used in a variety of applications, such as cleaning and etching metals, producing fertilizers, and in the production of pharmaceuticals.
Visit here to learn more about dilute acid brainly.com/question/30906166
#SPJ11
In the Fahrenheit temperature scale, water freezes at 32∘F and boils at 212∘F, in the Celsius scale, water freezes at 0∘C and boils at 100∘C, given that the Fahrenheit. temperature F and the Celsius temperature C are related by a linear equation, find F in terms of C. F( G )= Use your equation to find the Fahrenheit temperatures corresponding to 31∘C.21∘C,−9∘C, and −15∘C, to the nearest degree.
Answers
The Fahrenheit temperatures corresponding to Celsius 31°C, 21°C, -9°C, and -15°C are approximately 88°F, 70°F, 16° F, 5°F.
To find the equation relating Fahrenheit temperature (F) to Celsius temperature (C), we can use the given freezing and boiling points of water on both scales.
We know that at the freezing point of water:
Fahrenheit temperature = 32°F
Celsius temperature = 0°C
And at the boiling point of water:
Fahrenheit temperature = 212°F
Celsius temperature = 100°C
We can use these two points to find the equation of the line relating F and C.
First, we find the slope of the line:
Slope = (Change in Fahrenheit temperature) / (Change in Celsius temperature)
= (212°F - 32°F) / (100°C - 0°C)
= 180°F / 100°C
Next, we find the y-intercept of the line:
Using the freezing point of water (0°C, 32°F):
32°F = Slope * 0°C + y-intercept
32°F = 0.18 * 0°C + y-intercept
y-intercept = 32°F
Therefore, the equation relating Fahrenheit temperature (F) to Celsius temperature (C) is:
F = 1.8C + 32
Now, we can use this equation to find the Fahrenheit temperatures corresponding to the given Celsius temperatures:
For 31°C:
F = 1.8 * 31 + 32 = 87.8 ≈ 88°F
For 21°C:
F = 1.8 * 21 + 32 = 69.8 ≈ 70°F
For -9°C:
F = 1.8 * -9 + 32 = 15.8 ≈ 16°F
For -15°C:
F = 1.8 * -15 + 32 = 5 ≈ 5°F
Therefore, the Fahrenheit temperatures corresponding to 31°C, 21°C, -9°C, and -15°C are approximately 88°F, 70°F, 16° F, 5°F.
Learn more about temperatures from the link given below.
https://brainly.com/question/11464844
#SPJ4
A machine uses filtration to separate a component from orange juice. Which component does the machine most likely separate from the mixture?(1 point)
water
water
pulp
pulp
sugar
sugar
pigment
Which method of separation would be most appropriate for separating a mixture of water and alcohol?(1 point)
chromatography
chromatography
distillation
distillation
filtration
filtration
evaporation
Which method separates components of a mixture according to how quickly the particles travel through a medium?(1 point)
chromatography
chromatography
filtration
filtration
evaporation
evaporation
distillation
The boiling point of water is 100ºC. The boiling point of acetone is 56ºC. Which statement about distilling a mixture of acetone and water is correct?(1 point)
Acetone remains in the original container.
Acetone remains in the original container.
Acetone is captured and cooled.
Acetone is captured and cooled.
Water is collected as it leaves the mixture.
Water is collected as it leaves the mixture.
Water will vaporize from the mixture before acetone.
Substance A and substance B are mixed together. To separate the mixture, water is added, and substance A is filtered out. Then, the remaining liquid is heated to remove the water, leaving a residue of substance B. Which statement about substance A and substance B could be correct?(1 point)
Substance A is rice, and substance B is sugar.
Substance A is rice, and substance B is sugar.
Substance A is sand, and substance B is alcohol.
Substance A is sand, and substance B is alcohol.
Substance A is alcohol, and substance B is salt.
Substance A is alcohol, and substance B is salt.
Substance A is sugar, and substance B is instant coffee.
Answers
Pulp
distillation
chromatography
Acetone is captured and cooled.
Substance A is rice, and substance B is sugar.
To separate the components of a mixture the substances to be separated must differ in a given physical property.
In chemistry, there are diverse separation techniques used to separate the components of a mixture. Separation depends on differences in a given physical property such as boiling point, solubility in a solvent, difference in particle size etc.
Filtration can be used to separate a solid from a liquid hence it can be used to separate pulp from orange juice.
To separate water and alcohol, distillation is used because such a separation depends on the difference in the boiling points of water and alcohol. Water boils at a higher temperature than alcohol hence the mixture can be separated by distillation.
Chromatography is a method of separation that is dependent on how quickly a the components of a mixture move through a stationary phase.
Acetone has a lower boiling point than water hence it collected first and cooled before water.
Clearly, substance A must be a solid since it is filtered out after adding water. Substance B must be a water soluble substance which can be recovered from water by evaporation to dryness. Hence substance A is rice, and substance B is sugar.
https://brainly.com/question/11960023
help me please!!! this is very urgent !

Answers
Answer:
It is a wrong answer
• It's in theory that 1 hour = 60 minutes
\({ \tt{50 \: hours = (50 \times 60) \: minutes}}\)
• It's in theory that 1 minute = 60 seconds
\({ \tt{(50 \times 60) \: mins = \{(50 \times 60) \times 60 \} \: sec}}\)
» Therefore, correct expression is;
\({ \boxed{ \tt{50 \: hours = (50 \times 60 \times 60) \: seconds}}}\)
Which of the following should you multiply by in order to solve the following: 43 m = ? ft
Answers
Answer:
3.28
Explanation:
At some point it is best to write out an equation to cancel out the units you don't want and to get to the units that you do want. So if you write this out in an equation, you can see how you get to the answer.
43 m 3.28 ft ? ft
-------- X ----------- = --------------
1 1 m 1
You can see now that the meters on the top will cancel out the meters on the bottom so all you are left with is the feet unit. When you write out the equation you put the unit on the bottom that you want to get rid of (meter). You put the unit you want to get to on the top which is feet. At this point you just have to know how many feet 1 meter is equal to and plug those number in. 1 meter is equal to 3.28 feet.
someone pls explain how I do this work
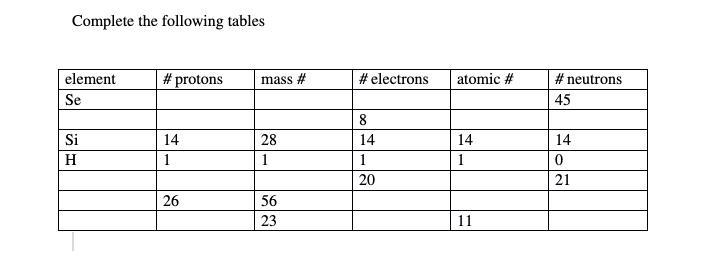
Answers
Answer:
Explanation:
Element #of protons Mass #. # of electrons Atomic #. Neutron
Se. 34. 78.96. 34 34 45
Si. 14. 28. 14. 14 14
H. 1. 1. 1 1 0
Ca. 20. 40.078. 20. 20. 21
Fe. 26. 56. 26. 26. 30
Na. 11. 23. 11. 11. 12
7. Given 483 g of Na2SO4. 10 H₂O, (a) Find the number of mole of Na+ and SO42-.
Answers
The number of moles of a substance is calculated from the given weight of the substance and its molecular mass by using the formula:
No. of moles = \(\frac{Mass of substance in grams}{Molecular mass of substance}\)
Molecular mass of Na₂SO₄.10H₂O is calculated to be
(23×2)+32+(16×4)+(10×18) = 46+32+64+180 = 322 g/mol
Thus, for 483g of Na₂SO₄.10H₂O, the number of moles would be
= \(\frac{322}{483}\) = 0.67 mol
For every 1 mole of Na₂SO₄.10H₂O, there are 2 moles of Na⁺ and 1 mole of SO₄²⁻.
Thus, 0.67 moles of Na₂SO₄.10H₂O will have 0.67×2= 1.34 moles of Na⁺ and 0.67 moles of SO₄²⁻.
Learn more about number of moles in:
https://brainly.com/question/14205424
#SPJ1
a solution is prepared by mixing 360.0 mL of 0.25 M NaOH, 140.0 mL of 0.50 M NaOH, and 300.0 mL of distilled water. Assuming that the volumes are additive, the molarity of NaOH in the resulting solution is
Answers
when was the last time a third-party candidate won any electoral votes?
Answers
The last third-party candidate to win one or more states was George Wallace of the American Independent Party in 1968.
Who was George Wallace?
Born in Clio, Wallace attended the University of Alabama School of Law and served in the United States Army Air Corps during World War II. After the war, he won election to the Alabama House of Representatives and served as a state judge.He first sought the Democratic nomination in the 1958 Alabama gubernatorial election. Initially a moderate on racial issues, Wallace adopted a hardline segregationist stance after losing the 1958 nomination.To know more about George Wallace, click the link given below:
https://brainly.com/question/480072
#SPJ4
1 pts
Define valence electrons.
o Valence electrons are the electrons on the outermost energy level of an
atom.
o Valence electrons are the electrons on the innermost energy level of an
atom.
Valence electrons are the electrons in the middle energy level of an
atom.
Valence electrons are the electrons on the inside of the nucleus of the
atom.
Answers
Answer:
Valence electrons are the electrons on the outermost energy level of an
atom.
The correct answer will be Valence electrons are the electrons on the outermost energy level of an atom.