what types of chemical bonds are present in a mixture of water (water molecules) and oil (triglyceride molecules)? what types of chemical bonds are present in a mixture of water (water molecules) and oil (triglyceride molecules)? covalent bonds and hydrogen bonds van der waals interactions, hydrogen bonds, and ionic bonds ionic bonds and van der waals interactions hydrogen bonds, van der waals interactions, and covalent bonds hydrogen bonds and van der waals interactions
Answers
The types of chemical bonds present in a mixture of water (water molecules) and oil (triglyceride molecules) are: Hydrogen bonds and van der Waals interactions.
Water molecules are polar and form hydrogen bonds with each other. These hydrogen bonds result from the attraction between the partially positive hydrogen atom of one water molecule and the partially negative oxygen atom of another water molecule.
Oil, on the other hand, primarily consists of nonpolar molecules such as triglycerides. Nonpolar molecules do not form hydrogen bonds but can interact through weak attractive forces known as van der Waals interactions. These interactions occur due to temporary fluctuations in electron distribution, resulting in induced dipoles that attract each other.
Therefore, in the mixture of water and oil, hydrogen bonds form between water molecules, while van der Waals interactions occur between oil molecules and potentially between water and oil molecules.
To know more about the chemical bonds refer here :
https://brainly.com/question/21106444#
#SPJ11
Related Questions
17. Substances created, or made, during a chemical reaction are known as the
b. products
d. substances
a. reactants
c. chemicals
Answers
Answer:
substances created or made during a chemical reaction is known as the products
Which of the following describes the properties of a
substance?
A. All of these
B. How it reacts with other chemicals.
C. How it looks.
D. How it behaves.
Answers
Answer:
B
Explanation:
The properties of a substance can be measured when matter undergoes a change.
The one that describes the properties of a substance is how it reacts with other chemicals, How it looks, and How it behaves. The correct option is A. All of these.
What are properties?The property of a substance is its unique quality and physical and chemical appearance. The properties of a substance are of two types: chemical and physical.
Physical properties talk about how a substance looks its color, its texture, and its solidness or softness. Some physical properties are density, color, melting, and boiling point.
Chemical properties are those properties that tell about how a substance reacts with other substances. Some chemical properties are reaction with or reaction with air.
Thus, the correct option is A. All of these, How it reacts with other chemicals, How it looks, and How it behaves describe the properties of a substance.
To learn more about the properties of a substance, refer to the link:
https://brainly.com/question/8930926
#SPJ5
A container holds three gases at a total pressure of 800 kPa. If the partial pressure of the first gas is 100 kPa and the partial pressure of the second gas is 300 kPa, what is the partial pressure of the third gas?
Answers
We can make use of the fact that the total pressure of the mixture is the same as the sum of the partial pressure of all the gases in the container. As a result, 400 kPa is the third gas' partial pressure.
What exactly is a partial pressure law?The overall pressure exerted by a mixture of gases is equal to the sum of the partial pressures of each of the constituent gases, according to Dalton's law of partial pressures. The partial pressure is the pressure that each gas would have if it occupied the same volume of the mixture at the same temperature on its own.
Mathematically, this can be stated as:
P_total = P_1 + P_2 + P_3
When we plug these figures into the formula, we get:
800 kPa = 100 kPa + 300 kPa + P_3
When we simplify this equation, we obtain:
800 kPa = 400 kPa + P_3
By taking away 400 kPa from both sides, we arrive at:
P_3 = 400 kPa
To know more about partial pressures visit:-
https://brainly.com/question/15075781
#SPJ1
What is the pH of a 0.0042 M LiOH solution?
Answers
The pH of a 0.0042 M LiOH solution can be calculated using the following steps:
1. Determine the concentration of the hydroxide ion (OH-) in the solution. Since LiOH is a strong base, it will completely dissociate in water, meaning the concentration of OH- will be equal to the concentration of LiOH:
[OH-] = 0.0042 M
2. Use the relationship between the concentration of H+ and OH- to find the concentration of H+:
Kw = [H+][OH-]
1.0 x 10^-14 = [H+](0.0042)
[H+] = 2.38 x 10^-12 M
3. Use the definition of pH to find the pH of the solution:
pH = -log[H+]
pH = -log(2.38 x 10^-12)
pH = 11.62
Therefore, the pH of a 0.0042 M LiOH solution is 11.62.
For more about pH:
https://brainly.com/question/15289741
#SPJ11
Which of the following is not considered a base unit according to the SI units of measurement

Answers
gram is not the SI unit of measurement. Thus option A is correct.
what is unit of measurement?
The unit of measurement can be defined as the magnitude of quantity that is used as the measurement for the same form, adopted and specified by the law or convention.
We can quantify different types of measurement by multiple measuring unit and this unit is called as the standard quantity of the physical property can be used as the factor for expressing the quantity of that specific.
There are apparent limitations of single unit of measurement like the use of the measurement of the same unit for the distance between two cities and the length of a pencil is impractical.
On the other hand length and weight, and volume are measured in different unit system like CGS system of units, the FPS system of units, the MKS system of units, and the SI system of units etc.
Learn more about SI unit, here:
https://brainly.com/question/12750330
#SPJ2
which form of energy does a cheeseburger have
Answers
Answer:
chemical potential energy - It mainly has chemical potential energy, this is really a type of electrical potential energy stored in the chemical bonds of the molecules
Explanation:
You can give the other person brainliest, I don't need it! :)
A sample of gas occupies a volume of 55.3 L, has a temperature of 23.3 °C and a pressure of .658 atm. Calculate the number of moles of gas which are present in the sample. R= .0821 atm L/mol K
Answers
Answer: The number of moles of gas which are present in the sample are 1.49 mol.
Explanation:
Given: Volume = 55.3 L
Temperature = \(23.3^{o}C\) = (23.3 + 273) K = 296.3 K
Pressure = 0.658 atm
Formula used is as follows.
PV = nRT
where,
P = pressure
V = volume
n = no. of moles
R = gas constant = 0.0821 L atm/mol K
T = temperature
Substitute the values into above formula as follows.
\(PV = nRT\\0.658 atm \times 55.3 L = n \times 0.0821 L atm/mol K \times 296.3 K\\n = \frac{0.658 atm \times 55.3 L}{0.0821 L atm/mol K \times 296.3 K}\\= \frac{36.3874}{24.32623}\\= 1.49 mol\)
Thus, we can conclude that the number of moles of gas which are present in the sample are 1.49 mol.
HELP PLEASE ASAP!! How many atoms are in 2.50mol of cobalt?
_________ atoms
Answers
One mole of cobalt contains 6.022 × 10²³ atoms and this number is called Avogadro number. Therefore, 2.50 mol of cobalt contains 15.05 × 10²³ atoms.
What is cobalt ?Cobalt is 27th element in periodic table. It is a transition metals and thus, classified into d-block elements. Its group members are rhenium and iridium.
Atoms are the basic units of every substance. Any substance containing 6.022× 10²³ atoms is called one mole of the substance.This number 6.022 × 10²³ is called Avogadro number. Therefore, One mole of every elements contains Avogadro number of atoms.
One mole of cobalt contains 6.022 × 10²³ or Avogadro number of atoms. Thus number of atoms in 2.50 moles of cobalt is calculated by multiplying the number moles by Avogadro's number.
The number of atoms 2.50 moles of cobalt = 2.50 × 6.022 × 10²³
= 15.05 × 10²³ atoms
Therefore, 2.50 moles of cobalt contains 15.05 × 10²³ atoms.
To find more about Avogadro number, refer the link below:
https://brainly.com/question/11907018
#SPJ1
Use the equation to answer the question.
H2O(l) + heat = H2O(g)
A sample of water is at equilibrium at 100°C. Which statement best describes what will happen if liquid water is added to the system?
A) All of the liquid water molecules that are added will remain
liquid water.
B) More water vapor molecules will change to liquid water until a
new equilibrium is reached.
C) All of the liquid water molecules that are added will become
water vapor.
D) More liquid water molecules will change to water vapor until a
new equilibrium is reached.
Answers
Answer:
D
Explanation:
More liquid water molecules will change to water vapor until a
new equilibrium is reached.
Answer:
Equilibrium and Stability Quick Check
1. The rate of the forward reaction equals the rate of the reverse reaction.
2. More LIQUID WATER molecules will change to WATER VAPOR until a new equilibrium is reached.
3. By adding WATER (H2O).
4. The additional BROMINE IONS cause the equilibrium to shift to the REACTANTS.
5. Removing WATER (H2O).
Explanation:
100% if you put these answers.
1. If a water body has large amount of suspended inorganic
materials, it will be__ in color.
a. Blue
b. yellowish to reddish, depending on the components of thr
inorganic materials.
c. Green
d. Clear
Answers
If a water body has large amount of suspended inorganic materials, it will be yellowish to reddish, depending on the components of the inorganic materials in color hence b. is the correct option.
A water body has all kinds of materials and compounds found in it which dictates it's color. If a water body has a large amount of suspended inorganic materials, the color will be affected by those materials. Depending on their components, the water may appear yellowish to reddish. This therefore rules out all the other options of blue, green and clear colours. The remaining correct option is b.
More on inorganic materials: https://brainly.com/question/6194734
#SPJ11
How many grams of lioh are needed to make 100 ml of a 0.1 m solution?
Answers
0.24 g of LiOH are needed to make 100 ml of a 0.1 m solution.
What is LiOH?Lithium hydroxide (LiOH), commonly obtained by the reaction of lithium carbonate with lime, is used in making lithium salts (soaps) of stearic and other fatty acids; these soaps are widely used as thickeners in lubricating greases.
Lithium hydroxide is an inorganic compound with the formula LiOH. It can exist as anhydrous or hydrated, and both forms are white hygroscopic solids. They are soluble in water and slightly soluble in ethanol. Both are available commercially.
LiOH (Lithium hydroxide) is a base. It is because it releases OH– (hydroxide-ion) in water. According to theories, a substance that can accept a proton or donate electrons or react with acid is classified as a base. LiOH reacts with strong acid HCl that produce LiCl and water.
Determination of mass of LiOH required:The molarity of LiOH is 0.1M
The given volume is,
M = W1 × 1000/m1 × V(ml)
m1 = molecular mass of LiOH = 24
putting the values in the previous equation,
0.1 = W1 × 1000/24 × 100
W1 = 0.1 × 24 ×100/1000
⇒ 0.24 grams of LiOH is required.
To learn more about LiOH refers to:
brainly.com/question/8053370
#SPJ4
which of the following statement is correct? liquid staTE HAS THE MOST ENERYGY
Answers
Answer:
no because gas has the most kinetic energy
when an aromatic ring is deactivated, what happens to the rate of an electrophilic aromatic substitution reaction?
Answers
The molecular dipole moments of the substituents, which are used to quantify their effect on electron donating or withdrawing, may be associated with the deactivation of the aromatic ring toward electrophilic substitution.
A substituted ring with an activating group undergoes the EAS reaction more quickly than benzene. A substituted ring with a deactivated group, on the other hand, moves more slowly than benzene. By using resonance or inductive electron donation, activating groups accelerate the EAS reaction. The reaction rate with regard to benzene is increased or decreased by the group(s) linked to the aromatic ring. the new substituent's entrance position. Activating and deactivating groups, respectively, affect the rate of the electrophilic aromatic substitution process with regard to benzene..
To know more about aromatic ring please click on the link brainly.com/question/14651665
#SPJ4
Can someone tell me the moles and atoms for MgN4(OSi2)3 and 3MgH2O3
Answers
The number of moles and atoms for MgN4(OSi2)3 is; 1mole and and 14atoms respectively.
The number of moles and atoms for 3MgH2O3 is; 3 moles and 18atoms respectively.
What is a mole of a substanceA mole put simply, is the base unit of amount of substance in the International System of Units. It is quantitatively defined as exactly 6.02214076×10²³ elementary entities, which may be atoms, molecules, ions, or electrons.
Hence, in MgN4(OSi2)3; since only one unit of the substance is given; Therefore, the number of moles is 1 and upon counting the number of individual atoms; the total number of atoms is; 14atoms
Similarly, in 3MgH2O3; since three units of the substance is given; Therefore, the number of moles is 3 and upon counting the number of individual atoms; the total number of atoms in one mole is 6 and in 3 moles is therefore; 18atoms.
Read more on Moles;
https://brainly.com/question/15356425
Which pH is the most acidic
Answers choices below
A. 7
B. 14
C. 0.5
D . 1
Answers
C. 0.5
This is the answer.
(assuming that the D option is 1, if it is 0.1 then the answer is 0.1)
\(0.5\)
Explanation:
As you know that decreasing in the concentration of OH- (i.e., 14, 13, 12, 11,..) makes to increase in the concentration of H+ ions (5,4,3,2,1).
what is the sequence of events from the arrival of an action potential at the synaptic knob until the release of neurotransmitter into the synaptic cleft?
Answers
The sequence of events at the neuromuscular junction is: 1. Arrival of the action potential at the synaptic knob, 2. Release of ACh into the synaptic cleft, 3.Binding of ACh to ACh receptors in the motor end plate, 4.Generation of action potential in sarcolemma, 5.Removal of ACh from the cleft by acetylcholinesterase
The action potential travels down the axon of the motor neuron and reaches the synaptic knob. This causes the release of the neurotransmitter acetylcholine (ACh) into the synaptic cleft. The ACh binds to ACh receptors in the motor end plate of the muscle fiber, which leads to the generation of an action potential in the sarcolemma (muscle cell membrane). The action potential then travels along the sarcolemma and deep into the muscle fiber, causing muscle contraction. Finally, the ACh in the synaptic cleft is rapidly broken down by the enzyme acetylcholinesterase to prevent continuous muscle contraction.
To know more neurotransmitter here
https://brainly.com/question/14256426
#SPJ4
How do weathering and deposition differ? Weathering breaks down rocks; deposition leaves them in new places. Weathering has to do with air; deposition has to do with plants. Weathering occurs only in summer; deposition occurs year-round. Weathering can be chemical or physical; deposition is only chemical
Answers
Answer:
A. Weathering breaks down rocks; Deposition leaves them in new places.
Explanation:
Weathering is basically the complete process of rocks breaking apart. In contrast, deposition is when the rocks are moved and carried away from their original place and put in new locations.
Answer:
a
Explanation:
What is meant by freezing point of a substance?
Answers
Answer:
It reaches a certain temperature that freezes the substance completely
The freezing point of a substance is the temperature at which it transitions from a liquid phase to a solid phase at a given pressure. It is the temperature at which the liquid and solid phases of a substance coexist in equilibrium. At the freezing point, the temperature of a substance remains constant until all of the liquid has been converted to a solid. Different substances have different freezing points, which can be used to identify the substance or to monitor its purity...
Formula for Gold (I) Nitride
Answers
Answer:
Gold(I) Nitride Au3N Molecular Weight -- EndMemo
What do you think the difference is between a personal characteristic and a physical description, when talking about people?
Answers
Answer:
I would say a physical characteristic is about what a person looks like, whereas a personal characteristic is how they act, and what theor personality is like.
Explanation:
Physical means something you can see or touch, like hair or skin. Personal is more often used for how someone's personality is, and you can't touch someone's personality.
he number of gas particles in a container will have the greatest impact on _____.
Answers
The number of gas particles in a container will have the greatest impact on pressure.
The number of gas particles in a container has the greatest impact on pressure because pressure is directly related to the frequency and magnitude of molecular collisions with the container walls.
When gas particles are in motion, they collide with each other and with the walls of the container. These collisions exert a force on the walls, which we perceive as pressure. The more gas particles there are in the container, the greater the number of collisions with the walls per unit of time.
According to the kinetic theory of gases, the pressure of a gas is proportional to the average kinetic energy and the frequency of collisions of the gas particles. Increasing the number of gas particles increases the frequency of collisions, resulting in more forceful and frequent impacts on the container walls. This leads to a higher pressure.
Conversely, if the number of gas particles decreases, there are fewer collisions occurring per unit of time, resulting in a lower pressure. Therefore, the number of gas particles in a container has the greatest influence on the pressure exerted by the gas inside.
To learn more about kinetic energy click here;
brainly.com/question/26472013
#SPJ11
a different student trying to determine if a different white solid is a true hydrate heats the sample and observes water droplets on the side of the test tube. the residue obtained is brownish and dissolves in water, producing a solution that is dark reddish-brown. is this a true hydrate? provide full reasoning.
Answers
Based on the observations described, it is likely that the white solid is a true hydrate.
Water droplets on the side of the test tube: When heating a hydrate, the water molecules trapped within the crystal lattice are released as vapor. The presence of water droplets on the side of the test tube indicates that water was indeed released during the heating process.
Brownish residue: The brownish residue obtained after heating the solid suggests that the white solid might contain a transition metal ion. Transition metal ions can form complex compounds that exhibit different colors, including brown.
Dissolving in water: The brownish residue dissolves in water, indicating that it is soluble in the solvent.
Dark reddish-brown solution: The solution obtained after dissolving the brownish residue is described as dark reddish-brown. This color could be attributed to the formation of a complex compound between the transition metal ion in the residue and the water or other substances present in the solution.
Based on the observations of water droplets upon heating, the brownish residue that dissolves in water, and the resulting dark reddish-brown solution, it is likely that the white solid is a true hydrate.
The presence of water droplets and the dissolution of the residue suggest that water was released from the solid during heating, indicating the presence of water molecules within the crystal lattice.
The color change to brownish and the subsequent dark reddish-brown solution point towards the involvement of a transition metal ion, possibly forming a complex compound.
To know more about hydrate visit:
https://brainly.com/question/29188417
#SPJ11
to generate (s)-mandelic acid, anion 1 should be protonated from where
Answers
Anion 1 should be protonated from the carboxylic acid group to generate (s)-mandelic acid. (S)-Mandelic acid is a chiral molecule that can be synthesized from racemic mandelic acid through an enantioselective resolution process. In this process, racemic mandelic acid is reacted with a chiral base, such as cinchonidine or tartaric acid, to form two diastereomers.
To generate (S)-mandelic acid from racemic mandelic acid, the anion 1 should be protonated from the carboxylic acid group. This protonation reaction converts the carboxylate anion (-COO-) into a carboxylic acid (-COOH) group, which is necessary for the enantioselective resolution process to occur. The protonation can be achieved using an acid such as hydrochloric acid (HCl) or sulfuric acid (H2SO4).
The carboxylic acid group is a functional group that contains a carbon atom that is double-bonded to an oxygen atom and single-bonded to a hydroxyl group (-COOH). In mandelic acid, there are two carboxylic acid groups, one at each end of the molecule. When mandelic acid is dissolved in water, the carboxylic acid groups can ionize to form carboxylate anions (-COO-), which have a negative charge. To convert the carboxylate anions into carboxylic acids, an acid can be added to the solution to donate a proton (H+). The protonation reaction results in the formation of a carboxylic acid group, which has a neutral charge. In the case of (S)-mandelic acid, the carboxylic acid group on the chiral center must be protonated to generate the (S)-enantiomer.
To know more about carboxylic visit :
https://brainly.com/question/31982037
#SPJ11
Investigation of Binding Behavior between Drug Molecule 5-Fluoracil and M4L4-Type Tetrahedral Cages: Selectivity, Capture, and Release,
Answers
the investigation of binding behavior between 5-Fluoracil and M4L4-type tetrahedral cages involves studying selectivity, capture, and release processes using techniques like molecular modeling and spectroscopy.
The investigation of binding behavior between the drug molecule 5-Fluoracil and M4L4-type tetrahedral cages involves studying the selectivity, capture, and release processes.
In this study, researchers aim to understand how 5-Fluoracil interacts with the tetrahedral cages and how these interactions affect the binding behavior. They may use techniques like molecular modeling, spectroscopy, and computational analysis to analyze the binding affinity and stability of the drug molecule within the cages.
To investigate selectivity, researchers may compare the binding behavior of 5-Fluoracil with other similar drug molecules or control compounds. This can help determine if the tetrahedral cages exhibit preferential binding towards 5-Fluoracil or if they can selectively capture and release the drug molecule.
During the capture process, the researchers will examine how the drug molecule enters the cavity of the tetrahedral cages and forms stable complexes. They may investigate factors like size, shape, and electrostatic interactions that contribute to the binding process.
Lastly, the release process involves understanding how the drug molecule is released from the tetrahedral cages. This could be triggered by changes in temperature, pH, or the presence of specific molecules. By studying the release mechanism, researchers can gain insights into the potential applications of these cages in drug delivery systems.
In conclusion, the investigation of binding behavior between 5-Fluoracil and M4L4-type tetrahedral cages involves studying selectivity, capture, and release processes using techniques like molecular modeling and spectroscopy.
Learn more about spectroscopy from the given link:
https://brainly.com/question/28543039
#SPJ11
Need help too please I would really appreciate it

Answers
Answer:
it's O2 since the oxygen has 6 valence electrons and it can make 2 covalent bonds
Please help I will give brainly.
Thank you so much
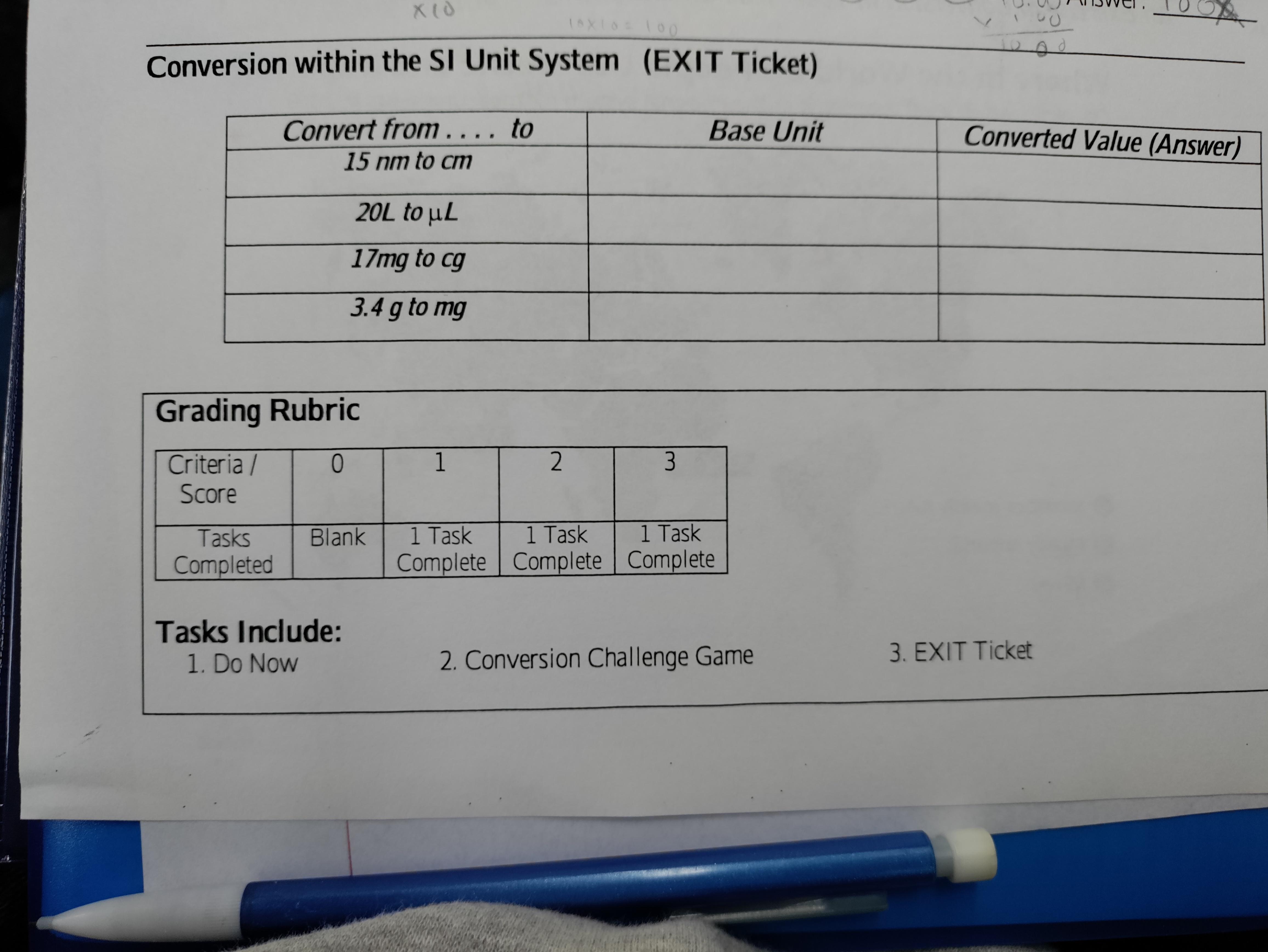
Answers
Answer:
meter. 1.5×10^-6
Litter. 2×10^-5
Kilo Gram. 1.7
Kilo Gram. 3.4 ×10^-3
I hope you understood that and if you don't understand write in comments
How many grams of au(iii) nitrate must be weighed in order to obtain exactly 3.00 grams of gold atoms?
Answers
Therefore, you would need to weigh approximately 5.16 grams of Au(III) nitrate to obtain exactly 3.00 grams of gold atoms.
To calculate the grams of Au(III) nitrate needed to obtain 3.00 grams of gold atoms, you'll need to consider the molar mass and stoichiometry.
First, find the molar mass of Au(III) nitrate (Au(NO3)3) by adding the atomic masses of each element:
Au (197 g/mol) + 3x N (14 g/mol) + 9x O (16 g/mol)
= 339 g/mol.
Next, determine the ratio of gold atoms to Au(III) nitrate in the balanced chemical equation. In this case, it's 1:1.
Now, divide the molar mass of Au(III) nitrate by the molar mass of gold atoms (197 g/mol) to get the ratio of grams of Au(III) nitrate to grams of gold atoms:
339 g/mol ÷ 197 g/mol = 1.72 g/mol.
Finally, multiply the ratio by the desired amount of gold atoms (3.00 g) to find the grams of Au(III) nitrate needed:
1.72 g/mol x 3.00 g = 5.16 grams.
Therefore, you would need to weigh approximately 5.16 grams of Au(III) nitrate to obtain exactly 3.00 grams of gold atoms.
To know more about gold visit:
https://brainly.com/question/20356891
#SPJ11
Solve for the following and reporting the correct number of significant figures and units.
Explain how the number of significant figures was determined. (1 pt ea)
a. 71.45 cm
-
135.2 mm - 44.7 cm =
Answers
The subtraction operation of the given figures is determined as 13.23 cm in 4 significant figure.
What is significant figure?A significant figure is a figure or a digit that contributes to how accurately something can be measured.
Also, significant figures are used to establish the number which is presented in the form of digits.
The given values;
71.45 cm ------> 4 significant figure
135.2 cm ---------> 4 significant figure
44.7 cm -------------> 3 significant figure
Perform subtraction operation in the given digitsSubtraction operator in mathematics is denoted by the symbol "-" and it implies subtracting the giving digits together.
Substitute the given values and subtract them as shown in the corresponding significant figures.
= 71.45 cm - 135.2 mm - 44.7 cm
= 71.45 cm - 13.52 cm - 44.7 cm = 13.23 cm (4 significant figure).
Thus, the subtraction operation of the given figures is determined as 13.23 cm in 4 significant figure.
Learn more about significant figures here: https://brainly.com/question/24491627
#SPJ1
Name the following aromatic hydrocarbon:
CH,CH;
CH,CH3
A. 1,4-dimethylbenzene
B. 1,4-dihexylbenzene
C. 2,6-diethylbenzene
D. 1,4-diethylbenzene
Answers
Answer:
A. 1,4-dimethylbenzene
Explanation:
The compound CH,CH;CH,CH3 is a benzene derivative with two methyl groups attached to the benzene ring. The positions of the methyl groups on the ring are indicated by the numbers 1 and 4, which correspond to the carbon atoms at the end of the two double bonds in the benzene ring. This compound is therefore 1,4-dimethylbenzene. The other options listed are not correct because they contain the wrong number or type of substituents on the benzene ring.
A potential problem with application of a multipurpose dry chemical agent to a class k fire is:_____.
Answers
While multipurpose dry chemical agents are effective for many types of fires, they may not be as effective for class K fires involving cooking oils and fats. Specialized wet chemical agents are often the preferred choice for tackling these fires due to their ability to cool, penetrate, and prevent re-ignition.
A potential problem with the application of a multipurpose dry chemical agent to a class K fire is that it may not effectively extinguish the fire or prevent re-ignition. Class K fires involve flammable cooking oils and fats, commonly found in commercial kitchens.
Multipurpose dry chemical agents are typically designed to extinguish class A, B, and C fires. They work by interrupting the chemical reactions that sustain the fire. However, when it comes to class K fires, the high temperatures and unique properties of cooking oils and fats can pose challenges.
One reason for the potential problem is that multipurpose dry chemical agents may not cool the burning cooking oils and fats enough to prevent re-ignition. These fires can reach extremely high temperatures, and if the agent doesn't effectively cool the fuel source, the fire may rekindle once the agent dissipates.
Another potential problem is that the dry chemical agent may not fully penetrate the cooking oils and fats to reach the base of the fire. The thick consistency of these fuels can create a barrier that prevents the agent from effectively reaching the core of the fire. This can result in incomplete extinguishment and the fire continuing to burn.
To address these challenges, specialized wet chemical agents are often used for class K fires. These agents are specifically formulated to cool the fuel source and create a foam-like blanket that prevents re-ignition. They have better penetration capabilities and are designed to handle the unique properties of cooking oils and fats.
Learn more about dry chemical agents here:-
https://brainly.com/question/31579854
#SPJ11