What volume of 0. 256 m sodium bromide contains 18. 7 grams of sodium bromide?.
Answers
The amount of sodium bromide is 18.7 grams. The concentration of sodium bromide is 0.256 M. We have to calculate the volume of the solution containing 18.7 grams of sodium bromide. Converting grams of sodium bromide to moles:18.7 g Na Br × (1 mole/102.9 g) = 0.182 moles Na Br.
Converting concentration from molarity to moles per liter:0.256 M Na Br = 0.256 moles Na Br/L Using the formula: C = n/V, where C = concentration, n = number of moles and V = volume, we have;0.256 moles Na Br/L = 0.182 moles Na Br/V.
Volume V = 0.182 moles Na Br/0.256 moles/L Volume V = 0.71 L or 710 ml, Therefore, the volume of 0.256 M sodium bromide containing 18.7 g of sodium bromide is 0.71 L or 710 ml.
To know more about sodium visit:
https://brainly.com/question/30878702
#SPJ11
Related Questions
a compound is found to contain 42.88 % carbon and 57.12 % oxygen by mass. what is the empirical formula for this compound?
Answers
The empirical formula for the compound containing 42.88% carbon and 57.1% oxygen by mass is Carbon Monoxide (CO).
To find the empirical formula for 42.8% carbon (C) and 57.1% oxygen (O)
First, find the molar mass of each component-
C = 12.010, O = 15.999
Converting to the moles :
C = 3.563, O = 3.568
Finding smallest mole value :
C = 3.563
Dividing all components by smallest value :
C = 1, O = 1.001
By rounding off -
C = 1, O = 1
Combining to get empirical formula : CO
Now, multiplying all by 1 ( molar mass of empirical formula/molar mass of sample = 28.01 / 28.034 ) to get molecular formula : CO
Since multiplied by 1, Therefore, the empirical formula and molecular formula are same.
To learn more about the empirical formula,
brainly.com/question/15169981
#SPJ4
difference between sodium ion and sodium atom.
(give in points not in paragraph)
Answers
Answer:
Explanation:
SODIUM ATOM;
SODIUM ATOM IS NEUTRAL
SODIUM ION;
IT IS A CHARGED SPECIE WITH A CHARGE OF +1
SODIUM ATOM:
THE NUMBER OF PROTONS AND ELECTRONS ARE SAME ie:11
SODIUM ION:
NUMBER OF PROTONS AND ELECTRONS ARE NOT SAME ie. ELETRON: 10, PROTONS:11
HOPE IT WILL HELP:)
_______________is a physical property of butter or any solid fat.
Answers
Answer- better
_______________
hope it will help you
is Sulfuric Acid soluble if placed in water
Answers
Answer: Yes, sulfuric acid is highly soluble in water.
Explanation:
please help me
The number of protons in carbon (C) is
A equal to the atomic mass.
B can be determined by adding the atomic number to the atomic mass.
C can be determined by subtracting the atomic number from the atomic mass.
D is equal to the atomic number.
Answers
Answer:
D
Explanation:
Just that. The atomic number is equal to the atomic mass.
Can someone help with question 6 ASAP

Answers
Answer:
A metalloid is a type of chemical element which has a preponderance of properties in between, or that are a mixture of, those of metals and nonmetals. There is no standard definition of a metalloid and no complete agreement on which elements are metalloids. Despite the lack of specificity, the term remains in use in the literature of chemistry.
A series of six elements called the metalloids separate the metals from the nonmetals in the periodic table. The metalloids are boron, silicon, germanium, arsenic, antimony, and tellurium. These elements look metallic; however, they do not conduct electricity as well as metals so they are semiconductors. They are semiconductors because their electrons are more tightly bound to their nuclei than are those of metallic conductors. Their chemical behavior falls between that of metals and nonmetals. For example, the pure metalloids form covalent crystals like the nonmetals, but like the metals, they generally do not form monatomic anions. This intermediate behavior is in part due to their intermediate electronegativity values. In this section, we will briefly discuss the chemical behavior of metalloids and deal with two of these elements—boron and silicon—in more detail.
Explanation:
i hope this helps you :)
what is the formula of calcium carbonate
Answers
Answer:
CaCO3... Answerrrr.....
Answer:
\(CaCO_{3}\)
Explanation:
This is because calcium is a metal that forms ions with a 2+ charge (as it has 2 electrons on its outer shell).
Carbonate ions are complex ions with a 2- charge, so with one carbonate and one calcium, the 2+ and 2- charges will cancel out.
What problems may global warming cause?
Answers
Answer:
Global warming stresses ecosystems through temperature rises, water shortages, increased fire threats, drought, weed and pest invasions, intense storm damage and salt invasion, just to name a few.
Explanation:
Global warming stresses ecosystems through temperature rises, water shortages, increased fire threats, drought, weed and pest invasions, intense storm damage and salt invasion, just to name a few.
6.022 x 10^23 atoms of calcium.
What is the mass?
Answers
Answer:
(6.02214179×1023) / one mole of substance. How many atoms are in a 3.0 g sample of sodium (Na)?
Explanation:
Answer:
Explanation:
Mass is 1.000
How does genetic variation play a role in evolution? I give Brainless
Answers
Answer:
Evolution is the process by which populations of organisms change over generations. Genetic variations underlie these changes
Explanation:
If a trait is advantageous and helps the individual survive and reproduce, the genetic variation is more likely to be passed to the next generation.
Brain-List?
The proton,neutron and electrons in the atom of the element represented by the symbol 231Y89 are:
A. 89,231 and 89
B. 142, 89 and 142
C. 89, 142 and 89
D. 89, 320 and 320
Answers
Answer:
Protons = 89
Neutrons = 142
Electrons = 89
Option C
Explanation:
Hello,
Were required to find the proton, neutron and electron of an element Y
The atomic mass of element Y is composed of the protons and neutrons while the atomic number is the exact amount of protons present in the element.
Element = ₈₉²³¹Y
Atomic number = 89
Number of protons = 89
Number of electrons = 89
Atomic mass = 231
Atomic mass = protons + neutrons
231 = 89 + neutrons
Neutrons = 231 - 89
Neutrons = 142
Therefore the number of protons is 89, number of neutrons is 142 and number of electrons is 89.
Note: for an electrically neutral atom, the number of protons must be equal to the number of electrons
what happens when hydrochloric acid (hcl) is added to a carbonate salt? observation reaction products
Answers
When hydrochloric acid (HCl) is added to a carbonate salt, a chemical reaction occurs, producing carbon dioxide (CO2) gas, water (H2O), and a chloride salt. This reaction is called an acid-carbonate reaction. The key observation during this reaction is the effervescence or bubbling due to the release of carbon dioxide gas.
In this process, the HCl donates a hydrogen ion (H+) that reacts with the carbonate ion (CO3 2-) present in the carbonate salt. The reaction results in the formation of carbonic acid (H2CO3), which then breaks down into water and carbon dioxide gas.
The remaining part of the carbonate salt combines with the chloride ion (Cl-) from the hydrochloric acid to form the corresponding chloride salt.
The general equation for this reaction can be written as:
2HCl(aq) + M2CO3(s) → 2MCl(aq) + H2O(l) + CO2(g)
Here, M represents the metal cation of the carbonate salt, such as sodium (Na), calcium (Ca), or potassium (K).
An observable sign of this reaction is the effervescence or bubbling due to the release of carbon dioxide gas. The solution may also become clearer as the carbonate salt dissolves and reacts with the acid.
For more such questions on hydrochloric acid, click on:
https://brainly.com/question/3229358
#SPJ11
A single atom of an element has 11 protons, 11 electrons, and 12 neutrons. Which element is it? a V b Na c Mg d Se
Answers
Answer:
B. Na
Explanation:
To identify an atom, you simply need to look at the number of protons. This atom has 11 protons. On the periodic table, you can see that the element with 11 protons is sodium (Na).
you add 100.0 g of water at 60.0°c to 100.0 g of ice at 0.00°c. some of the ice melts and cools the water to 0.00°c. when the ice and water mixture has come to a uniform temperature of 0°c, how much ice has melted?
Answers
The amount of water that melts when 100.0 grams of pure ice are combined with 100.0 grams uniform temperature of water heated to 60.0 degrees Celsius is 50.3 grams. To 0 degrees Celsius, the water has been cooled.
What does uniform temperature mean?The term "temperature uniformity" refers to the ability of an oven to keep the desired temperature consistently throughout all of its operating duration, not only in one area.
Describe consistent heating.1. Evenly distributed heat flow at the ground. If the grooves are deep or if the macroscopic constriction resist in the tube walls is high compared to the interfacial contact resistance there at joint between the outer and inner tubes, the heat flux over the land would be roughly uniform.
To know more about uniform temperature visit:
https://brainly.com/question/3127810
#SPJ4
The blending of one s atomic orbital and two p atomic orbitals produces A) three sp hybrid orbitals B) two sp2 hybrid orbitals C) three sp3 hybrid orbitals D) two sp' hybrid orbitals E) threesp2 hybrid orbitals
Answers
The blending of one s atomic orbital and two p atomic orbitals produces three sp hybrid orbitals.
When an s orbital and two p orbitals combine, they undergo hybridization to form three sp hybrid orbitals. This hybridization occurs when an atom is bonded to three other atoms in a trigonal planar arrangement. The process involves mixing one s orbital and two p orbitals to form three equivalent hybrid orbitals.
These sp hybrid orbitals have a linear shape with an angle of 180 degrees between them. The term "sp" indicates that the hybrid orbitals are a combination of one s orbital and one p orbital. This type of hybridization is commonly observed in molecules with triple bonds or in the central atom of trigonal planar molecules.
To learn more about orbital click here:
brainly.com/question/32355752
#SPJ11
Consider the following oxidation reactions and their equilibrium constants. Rank these metals from strongest to weakest reducing agent. Strongest reducing agent Weakest reducing agent Cu Cd Ni
Answers
Oxidation and reduction are important concepts in chemistry, and they often go hand in hand with redox reactions. Metals that can be oxidized are referred to as reducing agents, whereas those that can be reduced are referred to as oxidizing agents.
The metal that has the greatest tendency to be oxidized is the strongest reducing agent, while the one with the least tendency to be oxidized is the weakest reducing agent. Copper (Cu), cadmium (Cd), and nickel (Ni) are three metals that will be considered. The oxidations and their equilibrium constants are given below:
Cu → Cu2+ + 2 e− [Cu2+] / [Cu]2Cd
→ Cd2+ + 2 e− [Cd2+] / [Cd]2Ni
→ Ni2+ + 2 e− [Ni2+] / [Ni]2
Using the above given equations, equilibrium constants can be determined for these metals:
Cu: Kc = [Cu2+] / [Cu]2
Cd: Kc = [Cd2+] / [Cd]2
Ni: Kc = [Ni2+] / [Ni]2
The smaller the value of Kc, the more spontaneous the reaction. Hence, the metal with the smallest Kc would be the strongest reducing agent, and the metal with the largest Kc would be the weakest reducing agent. According to the above equations, the metals in order of strength from the strongest to the weakest reducing agent are as follows: Cu, Cd, Ni. Therefore, copper is the strongest reducing agent, cadmium is the second strongest reducing agent, and nickel is the weakest reducing agent.
To know more about spontaneous visit-
https://brainly.com/question/5372689
#SPJ11
clerice midter
The diagram below is the Bohr model of an atom.
Which best describes this atom?
OA. It has 6 electrons.
OB. It has a positive charge.
O c. It has 6 valence electrons.
OD.
has a full outermost energy level.
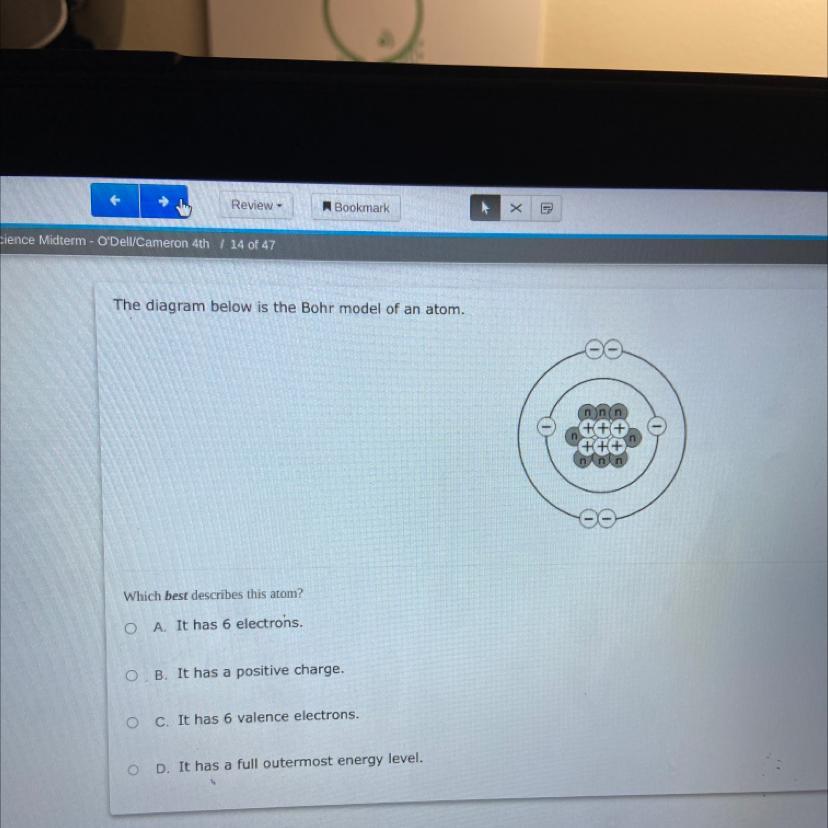
Answers
The correct option is (A) - This Bohr Model of atom describes that there are a total of 6 electrons in the given figure.
What is Bohr Model of atom?The electrons are positioned in circular orbitals at particular distances from the central nucleus in the Bohr model of the atom. These orbits create electron shells or energy levels, which allow us to see how many electrons are present in each shell. The number and the letter "n" are used to identify these energy levels. The first energy level nearest to the nucleus, for instance, is represented by the 1n shell. Normally, an electron resides in the shell with the lowest energy, which is the one closest to the nucleus. A photon of light's energy can raise it to a higher energy shell, but this is an unstable position, and the electron quickly returns to the ground state.
Learn more about atom here:
https://brainly.com/question/30898688
#SPJ1
Balance :FeCl3 + __Ca(OH)2 → ___ Fe(OH)3 + __ CaCl2
Answers
2. Predict the shift in the reaction with each stress shift rt, shift left, or no
HEAT + Ti(s) + 2C1 (g)
a. CI, (g) is added to the system.
b. TiCk (g) is removed from the system.
TiCI (g)
c. The temperature of the container is decreased.
d. The pressure of the container is increased.,
e. Ti(s) is added to the system.
Answers
b. The removal of TiCl(k) from the system will shift the reaction right, towards the products, to counteract the decrease in the concentration of TiCl(k).
c. Decreasing the temperature of the container will shift the reaction to the right, towards the products, to counteract the decrease in kinetic energy of the system.
d. Increasing the pressure of the container will shift the reaction to the left, towards the reactants, to counteract the increase in pressure caused by the decrease in volume.
e. Adding Ti(s) to the system will not cause a shift in the reaction because Ti(s) is not involved in the reaction.
List four greenhouse gases in the atmosphere. For each gas, describe its prevalence in the atmosphere, its natural sources, its human-induced sources, and how its concentration in the atmosphere might be changing.
Answers
Answer: Carbon Dioxide, Methane, Nitrous Oxide and Fuorinated Gases
Explanation:Carbon dioxide (CO2): Carbon dioxide enters the atmosphere through burning fossil fuels (coal, natural gas, and oil), solid waste, trees and other biological materials, and also as a result of certain chemical reactions (e.g., manufacture of cement). Carbon dioxide is removed from the atmosphere (or "sequestered") when it is absorbed by plants as part of the biological carbon cycle.
Methane (CH4): Methane is emitted during the production and transport of coal, natural gas, and oil. Methane emissions also result from livestock and other agricultural practices and by the decay of organic waste in municipal solid waste landfills.
Nitrous oxide (N2O): Nitrous oxide is emitted during agricultural and industrial activities, combustion of fossil fuels and solid waste, as well as during treatment of wastewater.
Fluorinated gases: Hydrofluorocarbons, perfluorocarbons, sulfur hexafluoride, and nitrogen trifluoride are synthetic, powerful greenhouse gases that are emitted from a variety of industrial processes. Fluorinated gases are sometimes used as substitutes for stratospheric ozone-depleting substances (e.g., chlorofluorocarbons, hydrochlorofluorocarbons, and halons). These gases are typically emitted in smaller quantities, but because they are potent greenhouse gases, they are sometimes referred to as High Global Warming Potential gases ("High GWP gases").
If you were running your own country, which energy resource would you choose for generating electricity? Think about cost, availability, safety, reliability, etc. Please explain your choice in 3-5 sentences
Answers
Answer:
See explanation
Explanation:
If I am running my country, I will go for biomass as an energy resource. Biomass is cheap and readily available. The use of biomass in electricity generation is largely safe and reliable; it even ensures a clearer environment!
Biopower plants burn biomass directly to produce high-pressure steam which is now used to drive a turbine generator to generate electricity. Biomass will always be available at almost no cost hence it offers an inexpensive energy resource for electricity generation.
why is it important to run a blank solution to set the zero %T for both Parts 1 and 11 in this experiment? How would your results be affected if you did not run a blank? 2. A student neglected to run the blank solution to set the zero %T in Part l and obtained the Beer's Law plot shown below. a. If the student used the plot as shown, how would their calculated values of Ke be affected b. How could the student modify their plot to improve their results? 0.8 0.7 0.6 0.5 0.4 0.3 0.2 0.1 0 0 10 20 30 40 concentration (M × 10°)
Answers
Running a blank solution is crucial in spectrophotometry experiments to establish the zero %T and account for background absorbance. Without running a blank, the results can be affected by systematic errors.
It is important to run a blank solution to set the zero %T in both Parts 1 and 2 of the experiment because it helps to account for any background absorbance or interference from the solvent or other components in the sample. Running a blank solution allows us to establish a baseline measurement of the solvent or the solution without the analyte, which helps in accurately measuring the absorbance caused by the analyte of interest.
If a blank solution is not run, the results can be affected in several ways:
Systematic Error: The absence of a blank solution can introduce a systematic error, causing a constant offset in the measured absorbance values. This offset can lead to incorrect calculations and interpretations.
Overestimation or Underestimation: Without running a blank, the measured absorbance may include contributions from the solvent or other interfering substances. This can lead to overestimation or underestimation of the analyte concentration, affecting the accuracy of the results.
Distorted Beer's Law Plot: In the absence of a blank, the plot obtained may not accurately represent the linear relationship between concentration and absorbance according to Beer's Law. This can lead to incorrect calculations of the slope (molar absorptivity) and affect the accuracy of future concentration determinations.
In spectrophotometry, the blank solution serves as a reference for setting the zero %T (transmittance) or absorbance value. By measuring the blank, we can account for any absorbance caused by the solvent, impurities, or other components in the sample. The blank solution typically contains all the components except the analyte of interest. It is measured under the same conditions as the sample solutions.
The blank measurement allows us to subtract any background absorbance from the sample measurements, providing a more accurate representation of the absorbance caused solely by the analyte. This helps in obtaining reliable and precise measurements for concentration determination using Beer's Law.
Running a blank solution is crucial in spectrophotometry experiments to establish the zero %T and account for background absorbance. Without running a blank, the results can be affected by systematic errors, inaccurate concentration determinations, and distorted Beer's Law plots. It is important to always include a blank solution to ensure accurate and reliable measurements.
To know more about spectrophotometry , visit:
https://brainly.com/question/24864511
#SPJ11
ider the a-module aε and show that every a-module homomorphism aε → aε is of the form x → xb for some b = εbε ∈ εaε.
Answers
Every a-module homomorphism aₑ → aₑ is of the form x → xb for some b = εbε ∈ εaε.
What is an a-module homomorphism?
An a-module homomorphism is a map between two a-modules that preserves the module structure, i.e., it respects the module addition and scalar multiplication.
To show that every a-module homomorphism aₑ → aₑ is of the form x → xb for some b = εbε ∈ εaε, let φ be an a-module homomorphism from aₑ to aₑ. For any x ∈ aₑ, we can express x as x = εa for some a ∈ a.
Then, φ(x) = φ(εa) = εφ(a). Let b = φ(a), so φ(x) = εb. Therefore, every a-module homomorphism aₑ → aₑ is of the form x → xb for some b = εbε ∈ εaε.
Therefore, the answer is that every a-module homomorphism aₑ → aₑ is of the form x → xb for some b = εbε ∈ εaε.
To know more about a-module homomorphism, refer here:
https://brainly.com/question/6111672
#SPJ4
Please help :/ || 10 pts || will mark brainlest ||
In which equation is carbon dioxide a product?
Group of answer choices
CH4 + 2O2 --> CO2 + 2H2O
CO2 --> C + O2
2H2O --> 2H2 + O2
6CO2 + 6H2O --> C6H12O6 + 6O2
Answers
Answer:
CH4 + 2O2 --> CO2 + 2H2O
Explanation:
"CO2" can be written as carbon dioxide
A 0.420 M Ca(OH)2 solution was prepared by dissolving 64.0 grams of Ca(OH)2 in enough water. What is the total volume of the solution formed? (4 points) a1.07 liters b1.23 liters c2.05 liters d2.18 liters
Answers
ANSWER
the volume of the solution is 2.05 liters
EXPLANATION
Given that;
The concentration of Ca(OH)2 is 0.420M
The grams of Ca(OH)2 is 64.0 grams
Follow the steps below to find the volume of the solution
Step 1; Calculate the number of moles of Ca(OH)2 using the below formula
\(\text{ mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, that the molar mass of Ca(OH)2 is 74.093 g/mol
\(\begin{gathered} \text{ mole = }\frac{\text{ 64}}{74.093} \\ \text{ mole = 0.864 mole} \end{gathered}\)Step 2; Find the volume of the solution in liters using the below formula
\(\text{ Molarity = }\frac{\text{ moles of the solute}}{\text{ liters of solution}}\)\(\begin{gathered} \text{ 0.420 = }\frac{\text{ 0.864}}{\text{ V}} \\ \text{ cross multiply} \\ \text{ 0.420V = 0.864} \\ \text{ Divide both sides by 0.420} \\ \text{ }\frac{\text{ 0.420V}}{0.420}\text{ = }\frac{\text{ 0.864}}{\text{ 0.420}} \\ \text{ V = 2.05 Liters} \end{gathered}\)Therefore, the volume of the solution is 2.05 liters
Bohr's model of the atom included a positively charged _____ orbited by negatively charged _____.
neutrons, electrons
electrons, protons
nucleus, protons
nucleus, electrons
Answers
Hope this helps *smiles*
Sorry if it’s wrong
If a liquid contains 60% sugar and 40% water throughout its composition then what is it called? Homogeneous mixture Solvent Heterogeneous mixture Compound
Answers
The liquid described, containing 60% sugar and 40% water throughout its composition, is called a homogeneous mixture.
A homogeneous mixture is a type of mixture where the components are uniformly distributed throughout the mixture, resulting in a uniform composition and appearance. In a homogeneous mixture, the components are not visibly distinct and cannot be easily separated by physical means, such as filtering or settling.
Homogeneous mixtures can be either single-phase or multi-phase, depending on the number of components present and the nature of their interactions. Examples of homogeneous mixtures include solutions (such as saltwater), alloys (such as brass), and some types of gels and emulsions.
Visit here to learn more about homogeneous mixture brainly.com/question/30587533
#SPJ11
How much of a sample remains after five half-lives have occurred?
1/5 of the original sample
1/25 of the original sample
1/32 of the original gample
1/64 of the original sample
Answers
The first half-life, we have 1 • 1/2 = 1/2 left.
After two: 1/2 • 1/2 = 1/4
Three: 1/4 • 1/2 = 1/8
Four: 1/8 • 1/2 = 1/16
And five: 1/16 • 1/2 = 1/32.
Answer:
1/32
Explanation:
Finish the equations (some cannot react, just write cnr)
Mg + .................. → Mg(NO3)2 + NO + H2O
Al + .................. → AlBr3 + H2
K2O + HNO3 → .................. + H2O
Ba + H3PO4 → .................. + H2
Ca + HBr → .................. + H2
NaOH + .................. → Na3PO4 + H2O
Fe2O3 + HNO3 → .................. + H2O
Mg + .................. → MgCl2 + H2
FeO + .................. → FeCl2 + H2O
Fe3O4 + H2SO4 → .................................... + H2O
Ba + HNO3 → .................. + NO2 + H2O
Na + HNO3 → .................. + NH4NO3 + H2O
MgO + HBr → .................. + H2O
KOH + .................. → K2SO4 + H2O
FeO + HNO3 → .................. + NO + H2O
NaOH + HCl → .................. + H2O
Answers
Answer:
Here are the finishes for each equation, respectively:
Mg + 4 HNO3 → Mg(NO3)2 + 2 NO + 2 H2O
2 Al + 6 HBr → 2 AlBr3 + 3 H2
K2O + 2 HNO3 → 2 KNO3 + H2O
Ba + H3PO4 → Ba3(PO4)2 + 3 H2
Ca + 2 HBr → CaBr2 + H2
3 NaOH + H3PO4 → Na3PO4 + 3 H2O
Fe2O3 + 6 HNO3 → 2 Fe(NO3)3 + 3 H2O
Mg + 2 HCl → MgCl2 + H2
FeO + 2 HCl → FeCl2 + H2O
Fe3O4 + 8 H2SO4 → 3 Fe2(SO4)3 + 8 H2O
Ba + 4 HNO3 → Ba(NO3)2 + 2 NO2 + 2 H2O
2 Na + 2 HNO3 → 2 NaNO3 + H2
MgO + 2 HBr → MgBr2 + H2O
2 KOH + H2SO4 → K2SO4 + 2 H2O
FeO + HNO3 → Fe(NO3)2 + NO + H2O
NaOH + HCl → NaCl + H2O
order: abc 150 mg. stock: abc 8% solution. how many ml(s) will you give? (round the answer to the nearest tenth)
Answers
You would need to give approximately 18.8 ml of the ABC 8% solution to administer a 150 mg dose.
To calculate the required amount in milliliters, we need to use the following formula:
(required amount in ml) = (required dose in mg) / (concentration of stock solution in mg/ml)
Here, the required dose is 150 mg, and the concentration of the stock solution is 8% or 8 mg/ml.
So, putting the values in the formula, we get:
(required amount in ml) = 150 mg / 8 mg/ml
(required amount in ml) = 18.75 ml
Rounding off the answer to the nearest tenth, we get:
(required amount in ml) ≈ 18.8 ml
Therefore, you would need to give approximately 18.8 ml of the ABC 8% solution to administer a 150 mg dose.
To learn about the solution:
https://brainly.com/question/30665317
#SPJ4