what word or two-word phrase best describes the shape of the nitrosyl chloride ( ) molecule?
Answers
The shape of the nitrosyl chloride (NOCl) molecule is best described as "bent" or "V-shaped."
To explain further, the nitrosyl chloride (NOCl) molecule has a central nitrogen (N) atom bonded to an oxygen (O) atom and a chlorine (Cl) atom.
According to the VSEPR (Valence Shell Electron Pair Repulsion) theory, the molecular geometry of a molecule depends on the repulsion between the electron pairs around the central atom. In the case of NOCl, the nitrogen atom has one lone pair of electrons and two bonding pairs (one with oxygen and one with chlorine).
This arrangement of electron pairs results in a bent or V-shaped geometry to minimize electron repulsion. The bond angles in NOCl are approximately 113 degrees, further confirming its bent or V-shaped molecular geometry.
To know more about VSEPR click on below link:
https://brainly.com/question/28775578#
#SPJ11
Related Questions
Hurry please!!! Lithospheric plates move because of the movement of the ___________________ under them.
a. lower mantle
b. asthenosphere
c. outer core
d. inner core
Answers
Answer:
A. Lower mantle
Explanation:
Answer:
lower mantle
Explanation:
the cage size of the zeolites is in the centimeters scale
True False
Answers
The statement "the cage size of the zeolites is in the centimeters scale" is False.
What is Zeolite?Zeolite is a crystalline and porous alumino-silicate mineral consisting of hydrated alkaline metals and alkali earth metals. These minerals have microporous structures that make them useful in industrial and medical applications, among other things. They have a diverse array of applications, including as catalysts, adsorbents, and molecular sieves. Zeolites are minerals that have a unique framework that is capable of trapping and holding a variety of molecules within their microporous structure.
Zeolites are small in size, typically between 0.3 and 2 microns. The cavities or pores within these crystals, known as cages, are in the range of 4 to 12 Angstroms in size (1 Angstrom = 0.1 nm). These cavities are small, which allows the zeolite to selectively filter molecules based on their size, shape, and chemical properties.}
Learn more about Zeolite: https://brainly.com/question/27174988
#SPJ11
Pick one answer do not put any links please

Answers
which statement regarding linoleic acid [18:2(δ9,12)] is false?
Answers
Linoleic acid [18:2(δ9,12)] is an essential polyunsaturated fatty acid that cannot be synthesized by the human body and must be obtained through diet. It plays a crucial role in maintaining the structural integrity of cell membranes and is a precursor to several important signaling molecules.
However, one false statement regarding linoleic acid is that it is only found in animal products. This is not true as it is predominantly found in vegetable oils such as soybean, sunflower, and corn oil.
Adequate intake of linoleic acid is necessary for optimal health, but excessive intake may have negative effects on inflammation and chronic disease risk.
It is recommended that adults consume 5-10% of their daily caloric intake from linoleic acid sources.
To know more about polyunsaturated please visit....
brainly.com/question/31939320
#SPJ11
Someone pls help me I will make you brain

Answers
Answer:
As shown in the attachment,
Explanation:

Sugar turns into black mass on heating Give reason
need fast
Answers
The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot. hope this helps
Sugar is made of carbon, hydrogen, and oxygen atoms. When heated over a candle, these elements react with the fire to turn into a liquid. The heat causes the sugar's atoms to combine with the oxygen in the air, forming new groups of atoms. Energy is released in this chemical reaction in the form of smoke and black soot.
Calculate the molar mass of CO2
Answers
The gold-foil experiment was used to discover which subatomic particle? Question 2 options: electrons protons ==> neutrons (my answer)
Answers
Answer:Proton, the gold foil experiment indicated that the nucleus has a positive charge.
What is forensic
science?
Answers
Answer:
"Forensic science is a critical element of the criminal justice system. Forensic scientists examine and analyze evidence from crime scenes and elsewhere to develop objective findings that can assist in the investigation and prosecution of perpetrators of crime or absolve an innocent person from suspicion."
Explanation:
Strictly from: https://www.justice.gov/olp/forensic-science#:~:text=Forensic%20science%20is%20a%20critical,an%20innocent%20person%20from%20suspicion.
Forensic science is any science used to aid in a criminal/legal investigation
t/f do not use oil-based products (vaseline, body lotions) because they destroy latex
Answers
True. Do not use oil-based products such as Vaseline and body lotions because they destroy latex. Latex is a natural rubber, and when it comes into contact with oil-based products, it reacts chemically.
This reaction causes latex to degrade and lose its elasticity, making it prone to breakage. Therefore, it is important to avoid oil-based products when using latex products, such as condoms, gloves, and other medical supplies. Instead, use water-based products that are safe to use with latex. Water-based products are gentle on the skin and do not react chemically with latex, making them ideal for use with latex products.
learn more about chemically
https://brainly.com/question/12145141
#SPJ11
For BrO−BrO−, enter an equation that shows how the anion acts as a base.
Express your answer as a chemical equation including phases.
Answers
The equation that shows how the BrO⁻ anion acts as a base is:
BrO⁻(aq) + H₂O(l) → HBrO(aq) + OH⁻(aq)
When the BrO⁻ anion acts as a base, it accepts a proton (H⁺) from water (H₂O) to form the conjugate acid HBrO (hypobromous acid) and hydroxide ion (OH⁻). This is represented by the following chemical equation:
BrO⁻(aq) + H₂O(l) → HBrO(aq) + OH⁻(aq)
In this reaction, BrO⁻ acts as a base by accepting a proton from water, resulting in the formation of the conjugate acid (HBrO) and hydroxide ion (OH⁻).
When the BrO⁻ anion acts as a base, it can accept a proton (H⁺) from a stronger acid to form the conjugate acid. Here is an example of BrO⁻ acting as a base with HClO (hypochlorous acid):
BrO⁻(aq) + HClO(aq) → ClO⁻(aq) + HBrO(aq)
In this reaction, the BrO⁻ anion accepts a proton (H⁺) from HClO, forming the conjugate acid HBrO. The resulting product is the ClO⁻ anion, which is the conjugate base of HClO.
To know more about base refer here
https://brainly.com/question/9748243#
#SPJ11
For strong electrolytes, i = number of per mole of solute dissolved. CaCl dissolves yielding three ions, one Ca ion and two Clions, thus i = (NH. ),P dissolves yielding four ions, three NH' ions and one Pion, thus i = "Colligative Properties Study Guide" by Montgomery College is licensed under CC BY 4. 0
Answers
The statement you provided refers to the determination of the van't Hoff factor (i) for strong electrolytes. The van't Hoff factor represents the number of ions produced per mole of solute dissolved in a solution.
For example, when calcium chloride (CaCl2) dissolves, it dissociates into three ions: one Ca2+ ion and two Cl- ions. Therefore, the van't Hoff factor (i) for CaCl2 is 3 because it produces three ions per mole of solute dissolved.
Similarly, when ammonium phosphate (NH4)3PO4 dissolves, it dissociates into four ions: three NH4+ ions and one PO43- ion. Thus, the van't Hoff factor (i) for (NH4)3PO4 is 4 because it yields four ions per mole of solute dissolved.
The van't Hoff factor is essential in various calculations related to colligative properties, such as boiling point elevation and freezing point depression, where it is used to account for the number of particles in solution.
learn more about electrolytes here
https://brainly.com/question/32477009
#SPJ11
Barium crystallizes in a body-centered cubic structure with a unit cell edge length of 584.28 pm. Calculate the atomic radius of barium in this structure. Report your answer with five significant figures.
Answers
The atomic radius of barium in a body-centered cubic structure is approximately 222.59 pm.
Given parameters:Edge length (a) = 584.28 pmIn a body-centered cubic structure, the relationship between the unit cell edge length and the radius of atoms in the structure is:r = (a√3)/(4)Where r = radius of the atoma = unit cell edge lengthThe formula above is gotten from considering the diameter of the body-centered cubic structure.
Since the atoms in the body-centered cubic structure touch each other at the center of the cube, the diameter of the atoms would be equal to the length of the diagonal of the unit cell multiplied by √3/4.Diagonal of the unit cell = a√3Therefore, diameter of the atom = a√3/4r = diameter/2r = (a√3)/(4*2)By substituting the values of a and simplifying, we have;r = (584.28 pm √3)/(4*2) = 222.59 pmTherefore, the atomic radius of barium in a body-centered cubic structure is approximately 222.59 pm.
learn more about atomic radius
https://brainly.com/question/13126562
#SPJ11
Based on its location on the periodic table, how many electrons does oxygen have in its
outer energy level? (2 points)
O 3
O4
O 5
O 6
Answers
Answer:
O 6
Explanation:
Oxygen has six valence electrons, two in the 2s subshell and four in the 2p subshell.
During break time, you bought a magnum ice cream to your crush to show your gratitude in helping her during the previous activity. You bought a pair of magnum even though you don't have enough money. Thanks to your best friend who is always there to support you in times of need. As you approach her with confidence, you saw her talking to the person you saw last time in the canteen. As you turned around and leave, she called you. You have no choice but to confront her even though you feel that your heart is filled with sorrow. Then suddenly she introduced her brother. Your face turns to read with delight and also wonder? You told her that you didn't see her brother in IG, YT,FB or even tweeter. (such amazing stalker you are) then she told you the whole story to the point that you forgot to give the magnum ice cream that melted for some times in your hand. What phase change is being undergone as the magnum ice cream melt?
Answers
Answer:
Solid---> Liquid
What type of reaction is the following: Ca + S --> CaS
Answers
Answer:
Combination reaction is taken during this reaction
Bonus question which of these is the smallest??
Answers
Answer:
Explanation:
Atoms
Which one of the following substances is an element?
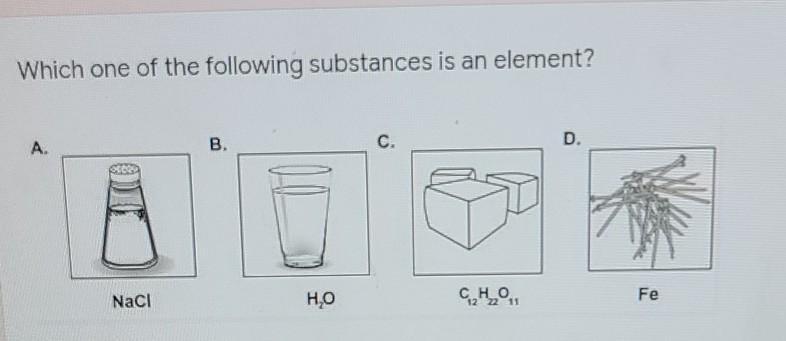
Answers
Answer: Fe
Explanation: Fe (Iron) is the only element since it involves only 1 atom. The other options are compounds, since there are more than 2 atoms bonded together.
What is the molar mass of methane (CHA)?
A. 16.05
grams
O B. 13.02 grams
O C. 49.05 grams
O D. 10 grams
Answers
an aqueous solution contains 15.2 ppm of dissolved cacl2. assuming the density of the solution is 1.00 g/ml, what is the concentration of cl− in ppm?
Answers
The concentration of Cl- in ppm in an aqueous solution containing 15.2 ppm of dissolved CaCl2 is 7.6 ppm. This is determined by using the formula [Cl-] = [CaCl2] / 2.
The concentration of a solute in a solution can be determined by the formula [solute] = (concentration in parts per million/density of solution) x 1000. Thus, the concentration of Cl- in the solution can be calculated as follows:
[Cl-] = (15.2 ppm/1.00 g/mL) x 1000 = 15200 mg/L
Since CaCl2 is composed of 1 mole of Ca2+ and 2 moles of Cl- ions, the concentration of Cl- in the solution is half of the concentration of CaCl2, or 7.6 ppm. This is the same as 7600 mg/L.
It is important to note that the concentration of a solute in a solution is dependent on both its parts per million (ppm) concentration and the density of the solution. Thus, if either of these factors change, the concentration of a solute in the solution will also change.
In summary, an aqueous solution containing 15.2 ppm of dissolved CaCl2 will have a concentration of Cl- of 7.6 ppm, determined by dividing the concentration of CaCl2 in parts per million by two and multiplying it by the density of the solution.
To learn more about concentration here:
https://brainly.com/question/17206790#
#SPJ11
Atoms of which elements form bonds without satisfying the octet rule?
O potassium (K) and sodium (Na)
O neon (Ne) and argon (Ar)
• nitrogen (N) and fluorine (F)
• helium (He) and hydrogen (H)
Answers
Option (D) helium (He) and hydrogen (H) is the right answer.
The term "chemical compound" refers to a combination of two or more chemical elements, whether they are similar or dissimilar. For instance, the chemical compound H2O is made up of two oxygen atoms and one hydrogen atom.Ionic, covalent, and hydrogen bonds are the three basic types of chemical bonds that are used to generate these chemical compounds from their component atoms.Helium and hydrogen are exceptions to this octet rule because they do not require 8 electrons to finish their while forming the bond with another element, the valence shell.Most elements from the periodic table generally follow the octet rule when forming the bond with the other elements, which is to complete their outermost shell with eight electrons.Hence we can consider that Option(D)helium (He) and hydrogen (H) is the right answer.To learn more about octet rule visit:
https://brainly.com/question/865531
#SPJ9
Answer: Helium, Hydrogen
Explanation: I got it right
suppose you burn a 2.5 g sample of potato chips. you use the heat given off from that process to heat 34.2 g of water from 17.4 oc to 20.7 oc. what is the caloric value (in kcal/g) of the potato chips?
Answers
0.05104 kcal/g is the calorific value of potato chips
Let the calorific value of potato be H cal/g
so, the heat of potato chips is 2.5g × H =2.5H
The specific heat of water is 1 cal g⁻1 °C⁻¹
The heat of water = 31.9 g × 1 cal g⁻1 °C⁻¹ ( 21.3-17.3)°C
= 31.9 × 4 cal
= 127.6 cal
hence, 2.5H = 127.6 cal
H = 127.6/2.5 = 51.4 cal/g = 0.05104 kcal/g
To know more about calorific value click here:
brainly.com/question/26854274
#SPJ4
Which compound has the lowest vapor pressure at 50C
Answers
Answer: Water
Explanation: In water, the hydrogen is bonded to an electronegative oxygen, and it can form hydrogen bonds. These are strong and thus keep the molecules together (molecules can not escape). Therefore, the compound with the lowest vapor pressure will be water.
the excess gibbs energy for the chloroform(1)/ethanol(2) system at 55°c is well represented by the margules equation the vapor pressures of chloroform and ethanol at 55°c are psat1
Answers
The excess Gibbs energy for the chloroform(1)/ethanol(2) system at 55°C can be accurately described by the Margules equation. This equation helps us understand the non-ideal behavior of the system by considering the interactions between the different components.
To determine the excess Gibbs energy, we need to know the vapor pressures of chloroform and ethanol at 55°C, which are denoted as psat1 and psat2, respectively. Unfortunately, the question seems to be cut off after mentioning psat1.
Without the complete information about psat1 and psat2, we cannot calculate the excess Gibbs energy or provide further insights into the system. If you have the complete data, please provide it so that we can proceed with the calculations.
In general, excess Gibbs energy quantifies the departure from ideality in a mixture and reflects the energy required to create or destroy intermolecular interactions between the components. It is an important concept in thermodynamics that helps us understand the behavior of mixtures and their phase equilibria.
Learn more about Gibbs energy on-
https://brainly.com/question/9179942
#SPJ11
PLEASE ANSWER ASAP
A classmate argues that the changes in energy that occur in a pendulum are unrelated to the energy changes that a burning log undergoes. She points out that the burning of a log is an irreversible chemical change in matter, while the energy changes in a moving pendulum are constantly reversing, and are examples of physical changes. Evaluate your classmate’s argument.
Answers
Answer:
The argument about the chemical and physical changes in the energy is correct.
This is so because when a log is burnt, the chemical change occurs as wood releases energy in the form of carbon dioxide are formed which is irreversible while the changes in energy of movement of the pendulum is a physical change as its potential energy converts to kinetic and kinetic energy again converts to the potential which is a reversible process.
Hence, the argument saying the changes in energy occurring on a pendulum and log is right.
For each of the following, highlight the element which has the highest:
Atomic Radius: F, Cl, Br
Ionization Energy: K, Ca, Sc
Electronegativity: C, Si, P, N
:) i'll give u brainliest
Answers
Ionization Energy: Sc (not sure about this one, couldn’t find it in the periodic table)
Electronegativity: N
Reason: Found electronegativity and atomic radius in my periodic table, wasn’t sure about ionization energy, so i hope i guessed right
Please help me with my science work

Answers
Answer:
what question do you need help with
Explanation:
Why does wood float besides it being less dense
Answers
Answer:
The only reason wood floats is because it is less dense and has big openings and gaps which allows air in.
Wood that sinks has very tiny openings. The ratio between weight and volume is called density. An object that is less dense than water can be held up by water, and so it floats.
Hope this helps! please mark me brainliest!
God bless :)
what color is this???

Answers
Answer:
I think it is yellow
Explanation:
Which method of heat transfer can take place without matter? O Conduction O Convection O Insulation O Radiation
Answers
Answer:
Radiation
Conduction is the transfer of thermal energy through direct contact. Convection is the transfer of thermal energy through the movement of a liquid or gas. Radiation is the transfer of thermal energy through thermal emission.