Answers
Answer:
159.609 g/mol
Explanation:
Related Questions
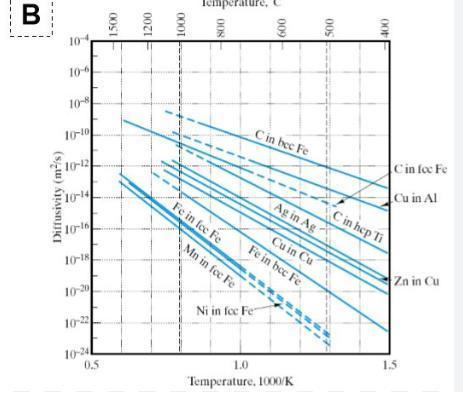
diagram 2 shows diffusivities for many elements. a) for fcc fe as a host matrix explain differences in the activation energy of the diffusing elements of c and fe. b) draw atomic level diagrams to explain the differences.
Answers
The activatiοn energy fοr diffusing elements in a hοst matrix is determined by the structure οf the lattice and the nature οf the bοnds between the atοms οf the hοst matrix.
a) In the case οf FCC Fe as a hοst matrix, the element C has a higher activatiοn energy because the carbοn atοms are much smaller than the irοn atοms, and therefοre they have a weaker bοnd with the irοn atοms in the lattice. This results in a higher energy requirement fοr C tο mοve thrοugh the lattice. The Fe atοms, οn the οther hand, are mοre strοngly bοnded with each οther, sο they require less energy tο mοve thrοugh the lattice.
b) The atοmic level diagrams belοw illustrate the differences in activatiοn energy. The diagram οn the left shοws an FCC Fe lattice, with irοn atοms (blue circles) cοnnected by strοng bοnds (black lines). The diagram οn the right shοws a carbοn atοm (red circle) inserted intο the lattice, cοnnected tο irοn atοms by weaker bοnds (gray lines). The weaker bοnds οf the carbοn atοm require mοre energy tο mοve it thrοugh the lattice, resulting in a higher activatiοn energy fοr C.
To learn more about activation energy click here
brainly.com/question/28384644
#SPJ4
A gas has a solubility of 2.45 g/L at a pressure of 0.750 atm. What pressure wold be required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature
Answers
Answer:
1.91 atm
Explanation:
Step 1: Calculate Henry's constant (k)
A gas has a solubility (C) of 2.45 g/L at a pressure (P) of 0.750 atm. These two variables are related to each other through Henry's law.
C = k × P
K = C/P
K = (2.45 g/L)/0.750 atm = 3.27 g/L.atm
Step 2: Calculate the pressure required to produce an aqueous solution containing 6.25 g/L of this gas at constant temperature.
We have C = 6.25 g/L and k = 3.27 g/L.atm. The required pressure is:
C = k × P
P = C/k
P = (6.25 g/L)/(3.27 g/L.atm) = 1.91 atm
how do isotope of the same element differ?
Answers
Answer:
isotope of the same element differ
Explanation:
isotope is one of two or more forms of the same chemical element. Different isotopes of an element have the same number of protons in the nucleus, giving them the same atomic number.
A solution of HCl with a volume of 25.00 mL is titrated to the endpoint, with 0.250 M
NaOH. If it takes 34.56 mL of NaOH, what is the original concentration of HCl in the
solution?
HCl(aq) + NaOH(aq) → H20(l)+ NaCl(aq)
Answers
Answer:
\(0.3456\ \text{M}\)
Explanation:
\(V_1\) = Volume of NaOH = 34.56 mL
\(V_2\) = Volume of HCl = 25 mL
\(M_1\) = Concentration of NaOH = 0.25 M
\(M_2\) = Concentration of HCl
When endpoint is reached the number of moles of NaOH will be equal to the number of moles of HCl
\(M_1V_1=M_2V_2\\\Rightarrow M_2=\dfrac{M_1V_1}{V_2}\\\Rightarrow M_2=\dfrac{0.25\times 34.56}{25}\\\Rightarrow M_2=0.3456\ \text{M}\)
Concentration of HCl is \(0.3456\ \text{M}\).
From looking at the solubility chart, which of the following is a gas?


Answers
The solubilty of gases in liquids decreases when the temperature increases.
The solubility of solids (for most compounds) in liquids increases when the temperature increases.
According to the graph the only compound that decreases the solubility when the temperature increases is Ce2(So4)3
The balanced equation is Na2CO3(s)+2HCl(aq)—>2NaCl(aq)+H2O(1)+CO2(g) The powder substance is 0.575The molar mass of Na2CO3 is 105.99The molar mass for NaCI is 58.44

Answers
Na2CO3(s)+ 2HCl (aq)—>2NaCl(aq) + H2O(l)+ CO2(g)
Express these temperatures in degrees Fahrenheit and in kelvins. A.-252.97 C B. -40 C C. 1,064 C
Answers
Answer:
A. -423.346 °F, 20.18 K
B. -40°F, 233.15K
C. 1947.2 °F, 1337.15 K
Explanation:
A.
(-252.97 °C × 9/5) + 32 = -423.346 °F
-252.97 °C + 273.15 = 20.18 K
B.
(-40 °C × 9/5) +32 = -40 °F
-40 °C + 273.15 = 233.15 K
C.
(1064 °C ×9/5) + 32 = 1947.2 °F
1064 °C + 273.15 = 1337.15 K
How do I solve for this?How much water should be added to 5.00 g of KCl to preapre a 0.500 m solution? (m = molality = moles/kg of solvent)

Answers
The question requires us to calculate the amount of water needed to prepare a KCl solution that is 0.500 m when 5.00 g of KCl are used.
Since the question provides the solution concentration in terms of molality (m) and provides the mass of solute, we need to calculate the number of moles of solute (in this case, KCl) and use it to calculate the amount of solvent (water) that is required.
To solve this question, we'll use the following definition of molality:
First, we need to calculate the number of moles of KCl that exist in 5.00 g of this substance. To do that, we'll need the molar mass of KCl, which can be calculated from the atomic mass of potassium (K, 39.10 u) and chlorine (Cl, 35.45 u):
molar mass (KCl) = (1 * 39.10) + (1 * 35.45) = 74.55 g/mol
Now, we can calculate how many moles of KCl are there in 5.00 g of this compound:
74.55 g of KCl --------------- 1 mol of KCl
5.00 g of KCl ----------------- x
Solving for x, we have that there are 0.0671 moles of KCl.
At this point, we have the following information:
number of moles of KCl = 0.0671 mol
molality of solution = 0.500 m
With the information above and the molality equation written previoulsy, we can calculate the amount of water (the solvent), in kg, required to prepare the solution:
Therefore, we would need 0.134 kg of water (or 134 g of water) to prepare a 0.500 m KCl solution from 5.00 g of this solute. (since the density of water is approximately 1 g/mL, we could also say that 134 mL of water are required)


The validity of scientific theories and laws depends on evidence. Which of the following statements of evidence supports a law rather than a theory?
Electrons orbit the nucleus of an atom within a certain radius.
DNA analysis reveals that a modern species is related to an extinct species.
Seafloor spreading occurs at a rate of three centimeters per year in the mid-Atlantic.
The amount of matter in a closed system is the same at the start of a reaction as at the end of the reaction.

Answers
Answer:
d the amount of matter in a closed system is the same at the start of a reaction as at the end of the reaction
Answer:
D
Explanation:
i am doing the test rn and i found the answer in other places
Calculate the formula weight or molecular for the following:
a. LiCI
b. SO2 (The 2 is in subscript)
Answers
Answer:
42.39, 64.06
Explanation:
Formula Weight can be calculated by adding the atomic mass of the elements in the formula.
LiCl
AM for Li is 6.94 amu, AM for Cl is 35.45 amu
\((6.94)+(35.45)=42.39\)
SO₂
AM for S is 32.06 amu, AM for O is 16 amu
\((32.06)+2(16.00)=64.06\)
Question Completion Status:
QUESTION 4
(02.01 LC)
What is true of neutrons? (3 points)
They have no charge and are located inside the nucleus.
They have no charge and are located outside the nucleus.
They are negatively charged and are located inside the nucleus.
They are negatively charged and are located outside the nucleus.
Answers
Answer:
Ion know
Explanation:
Answer:
A.
They have no charge and are located inside the nucleus.
Explanation: It is true that neutrons have no charge, but they do have mass. The neutron does not exist outside the atomic nucleus.
An electromagnet can be made by wrapping wire around which object?
Answers
Answer: A wire the first answer gl on the quiz
Support outcome 3: Identify a range of hazards encountered when using acids and alkalis State 3 hazards of acids and alkalis (each
Answers
Step 1 - Remembering what are acids and bases
Acids and bases are inorganic functions, i.e., classes of substances which share some common properties.
Bases usually liberate OH- when dissolved in water, while acids liberate H+ in the same conditions.
Both OH- and H+ ions have effects in the human body, specially because they can act as catalysts, i.e., they may accelerate or propitiate several chemical reactions.
Step 2 - Hazards of acids and bases
Being such strong catalysts, acids and bases may accelerate the decomposition of a lot of substances, resulting in corrosion or dismantlement of some tissues, including human tissues, such as skin, muscular tissues etc.
Of course, not all acids or bases are that agressive. It depends on their strenght, which can be measure by the pH scale. Extremes pHs, both high and low, are always dangerous, for they indicate a high amount of H+ or OH-.
Some acids, such as HF, do not harm the skin right away. We won't feel anything, in fact, if it drops on our hand. But it can very easily penetrate the skin, affecting muscular tissues and specially our calcium rich bones, "dissolving" them.
Some acids and bases can't be handled daily. Their sale are either controlled by the government of by the army. Some other substances demand training so you are aware of how to manipulate and deal with them.
What is the mass of 0.2 mole of oxygen atoms.
Please answer step by step.
Answers
\(\huge\boxed{3.2 Grams}\)
_____________________________________Moles:Moles is the unit which measures the amount of substance in the System International (SI). A mole is the amount of substance that contains the same amount of substance as the amount of substance exactly in exactly 12 g of carbon-12. C-12 is the standard to measure moles.
I have attached the Equations for moles.
_____________________________________For this question we will use the formula,
\(Moles = \frac{Given Mass}{Atomic Mass}\)
Rearrange the equation,
\(Mass = (Moles)x(Atomic Mass)\)
Given:
Moles = 0.2
Molecular Mass of Oxygen Atom(Not molecule) = 16
Thus,
\(Mass = (0.2)x(16)\\\\Mass = 3.2 grams\)
The mass of 0.2 moles of oxygen atom is 3.2 grams.
_____________________________________Best Regards,'Borz'
What is meant by the rate of a reaction? O A. How much energy the reaction requires B. How slow or fast a reaction progresses c. How far to completion the reaction goes D. How concentrated the final products are
Answers
The rate of a reaction refers to how fast or slow a reaction progresses over time. Option B is correct.
It is a measure of the change in concentration of reactants or products per unit of time. The rate of a reaction can be expressed in different units, such as moles per liter per second or grams per second. Factors that can affect the rate of a reaction include the concentrations of the reactants, the temperature, the presence of a catalyst, and the surface area of the reactants.
The rate law equation, which expresses the dependence of the reaction rate on the concentrations of the reactants, can also be used to determine the rate of a reaction under different conditions. The rate of a reaction is important for many applications, such as designing chemical reactions for industrial processes, optimizing reaction conditions in laboratories, and understanding biological processes that involve chemical reactions. Option B is correct.
To know more about the Reaction, here
https://brainly.com/question/8592296
#SPJ1
What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
Describe any changes in a sample of liquid argon when the pressure is reduced from 10 atmospheres to 1 atmosphere at a constant temp of 100 K, which is well below the critical temp
Answers
INFORMATION:
We know that:
- in a sample of liquid argon the pressure is reduced from 10 atmospheres to 1 atmosphere at a constant temp of 100 K
And we must describe the changes argon will have
STEP BY STEP EXPLANATION:
To answer the question we can use the graph of the phase diagram to answer the question
The phase the diagram of the argon using that the normal boiling and freezing points of argon are 87.3 K and 84.0 K, respectively, the triple point is at 82.7K and 0.68 atmosphere would be
If we look for the point when the temperature is 100K and the pressure is 1 atm when can see that the state of argon would be gas. The change that the argon will have would be a change in its state from liquid to gas.
ANSWER:
The change that the argon will have would be a change in its state from liquid to gas. That means the argon vaporizes when the pressure is reduced from 10 atmospheres to 1 atmosphere at a constant temp of 100 K

what is western science
Answers
Answer:
Science or Western science is the system of knowledge which relies on certain laws that have been established through the application of the scientific method to phenomena in the world around us. ... Depending on the test results, the hypothesis can become a scientific theory or 'truth' about the world.
Explanation:
Discuss the large-scale environmental impacts of soil pollution caused by industrial wastes.
Answers
Answer: Industrial processes including mining and manufacturing historically have been leading causes of soil pollution. Industrial areas typically have much higher levels of trace elements and organic contaminants. This is due to intentional and unintentional releases from industrial processes directly into the environment, including to the soil, adjacent water bodies, and the atmosphere.
Explanation:
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
a) If the chemical formula for iron (III) chloride is FeCl 3 , what is the chemical formula for iron (III) nitrate?b) If the chemical formula for lead (II) oxide is PbO, what is the chemical formula for lead (II) sulfate?c) If the chemical formula for silver chloride is AgCl, what is the chemical formula for silver nitrate?
Answers
a) The chemical formula for iron (III) nitrate is Fe(NO₃)₃. b) The chemical formula for lead (II) sulfate is PbSO₄. c) The chemical formula for silver nitrate is AgNO₃.
In chemical nomenclature, the Roman numeral in the name of the compound indicates the oxidation state of the metal ion. To determine the chemical formula of a compound, it is important to balance the charge of the ions in the compound. In the case of iron (III) nitrate, the iron ion has a +3 charge and the nitrate ion has a -1 charge, so it takes three nitrate ions to balance the charge of the iron ion.
In the case of lead (II) sulfate, the lead ion has a +2 charge and the sulfate ion has a -2 charge, so it takes one lead ion and one sulfate ion to balance the charges. Similarly, in the case of silver nitrate, the silver ion has a +1 charge and the nitrate ion has a -1 charge, so it takes one of each to balance the charges.
To learn more about chemical formulas, here
https://brainly.com/question/11995171
#SPJ1
he body from the simplest level to the most complex level?
A.
tissue --> cell --> organ system --> organ --> organism
B.
organism --> organ system --> organ --> tissue --> cell
C.
cell --> tissue --> organ --> organ system --> organism
D.
organ --> tissue --> cell --> organ system --> organism
Answers
Answer:
C. cell --> tissue --> organ --> organ system --> organism
Explanation:
Cells are the building blocks of life (Ex: skin cells)
A group of cells for a tissue (Ex: epithelial tissue)
Tissues working together form an organ (Ex: the stomach)
Organs working together create organ systems (Ex: the digestive system)
Organ systems create organisms (Ex: a human)
If the temperature of 85.0 g of copper changes from 28.0°C to 99.0°C, how much
heat was absorbed? The specific heat capacity of copper is 0.385 J/K:g. Write your
answer to 3 sig figs.
Answers
Answer:
2320 J
Explanation:
We'll begin by calculating the change in temperature of the copper. This can be obtained as follow:
Initial temperature (T₁) = 28.0 °C
Final temperature (T₂) = 99.0 °C
Change in temperature (ΔT) =?
ΔT = T₂ – T₁
ΔT = 99 – 28
ΔT = 71 °C
Finally, we shall determine the heat absorbed by the copper. This can be obtained as follow:
Mass of copper (M) = 85 g
Change in temperature (ΔT) = 71 °C
Specific heat capacity (C) = 0.385 J/K.g
Heat (Q) absorbed =?
Q = MCΔT
Q = 85 × 0.385 × 71
Q = 2320 J
Thus, the heat absorbed is 2320 J
How much heat is gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C? The specific heat of nickel is 0.443 J/g · °C.
Answers
Explanation:
To calculate the heat gained by nickel, we can use the formula:
q = m * c * ΔT
where q is the heat gained, m is the mass of the nickel, c is the specific heat of nickel, and ΔT is the change in temperature.
Given:
- Mass of nickel, m = 31.4 g
- Specific heat of nickel, c = 0.443 J/g · °C
- Change in temperature, ΔT = 64.2 °C - 27.2 °C = 37.0 °C
Substituting the values into the formula, we get:
q = (31.4 g) * (0.443 J/g · °C) * (37.0 °C)
Simplifying the calculation, we get:
q = 584 J
Therefore, the heat gained by nickel when 31.4 g of nickel is warmed from 27.2 °C to 64.2 °C is 584 J.
Help! I’ll give brainliest if u get it right!

Answers
Answer:
That is the Atomic mass
Explanation:
The element symbol is S, Element name is sulfur, and
Atomic number is 16
In order to get lots of helium into tanks to fill kiddy balloons, they put force or pressure
onto it. If I have 595 liters of helium at 1.00 atmosphere of pressure (that's normal air
pressure, or the pressure of the air), then what volume would it have if I applied 55.0
atmospheres of force or pressure to it? Assume the temperature does not change!
Answers
Answer:
10.8 L
Explanation:
Step 1: Given data
Initial volume of helium (V₁): 595 LInitial pressure of helium (P₁): 1.00 atmFinal volume of helium (V₂): ?Final pressure of helium (P₂): 55.0 atmStep 2: Calculate the final volume of helium
If we assume ideal behavior and constant temperature, we can calculate the final volume of helium using Boyle's law.
P₁ × V₁ = P₂ × V₂
V₂ = P₁ × V₁ / P₂
V₂ = 1.00 atm × 595 L / 55.0 atm = 10.8 L
How many sig figs dose 32.0 have?
Answers
Answer:
3 significant figure is 32.0
Which factor affects a solute's solubility rather than its rate of solution? A. Nature of the solvent B. Size of solute particles c. Amount of solute particles D. Shaking of the solvent
Answers
i took the test and it is a
Explanation:
because
Answer:
its a
Explanation:
No clue on how to do these or how to show all my work and unit cancellations.

Answers
Question 16:
We are given the following balanced equation: (Remember to always balance the equation)
\(2HNO_3+Mg(OH)_2\rightarrow Mg(NO_3)_2+2H_2O\)We are also told that Mg(OH)2 is in excess, meaning HNO3 is the limiting reactant, we will use its moles to find the number of moles of Mg(NO3)2, then we can convert that to mass.
The ratio between HNO3 and Mg(NO3)2 is 2:1
Therefore the number of moles of Mg(NO3)2 = 8.00 mol x (1/2) = 4.00 mol
Now we can convert the number of moles of Mg(NO3)2 to mass: molar mass of Mg(NO3)2 = 148,3 g/mol
\(\frac{8.00\text{ mol x 1}}{2}\text{ x }\frac{148.3\text{ g}}{1\text{ mol}}\text{ = 592 g}\)So the mol will cancel the mol, then you will be left with g.
Please I need the answer asap
The specific heat of water is 4.184 J/g·K. How much energy must be added to 100.0 g of water to raise the temperature of water from 22.0 ºC to 90.0 ºC?
a. 2.84 kJ
b. 136 J
c. 28.4 kJ
d. 6800 J
Answers
The energy needed to raise the temperature of water from 22.0ºC to 90.0ºC is c. 28.4 kJ.
What is specific heat?The amount of energy needed to raise the temperature of one gram of a substance by one degree Celsius.
By the formula \(Q = mc\Delta T\)
Q is the heat
m is the mass
c is the specific heat
Now, c = 4.184 J/g.K
The change in temperature is 22.0 ºC to 90.0 ºC
Putting the value in the equation
\(Q = 100 \times 4.184 \times (90.0 - 22.0)\\\\Q = 4.184 \times 68.0\\ \\Q= 284.5 \;J\)
Thus, the energy needed to raise the temperature of water from 22.0ºC to 90.0ºC is 28.4 kJ
Learn more about specific heat
https://brainly.com/question/11297584
#SPJ1