When comparing two sets of data, which one is more precise? Select one: A) The one with the smaller standard deviation B) The one with the mean further from the known value. C) The one with the mean closer to the known value D) The one with the larger standard deviation
Answers
The data set with the smallest standard deviation is the more accurate one.
standard deviation Smaller values of the standard deviation indicate less variability and higher precision. It measures the spread or variability of the data. The data points are more closely spaced and hence more tightly grouped around the mean when the standard deviation is less.The data's mean (average), on the other hand, is a measure of central tendency rather than precision. It is not always the case that greater or lesser precision is indicated by the mean being farther or closer than the known value.The response that has the smaller standard deviation
Which of the two standard deviation categories are they?Standard deviations come in two flavors: sample standard deviation and population standard deviation. Both gauge a set's level of dispersion. Nevertheless, the sample standard deviation only computes values that make up a portion of the overall data set, whereas the population computes all the values in a data set.learn more about standard deviation here
https://brainly.com/question/475676
#SPJ1
Related Questions
Which formula represents an unsaturated hydrocarbon?
1. C₂H4
2. C3H8
3. C4H10
4. C3H12
Answers
Answer:
1. C₂H4
Explanation:
The only saturated hydrocarbons are Alkanes: From that we can see that: C3H12, C3H8 and C4H10 are saturated. Therefore: C2H4 is unsaturated
why chromium acetate is diamagnetic
Answers
Chromium acetate is diamagnetic because it does not have any unpaired electrons in its orbitals, which are required for magnetic behavior.
Diamagnetic materials are materials that are not attracted to magnetic fields, and they behave in a manner that is opposite to magnetic materials, known as paramagnetic materials.
Chromium acetate has a complete electron shell configuration with all electrons paired, which means that the electrons are paired in the orbitals, and there is no net magnetic moment.
As a result, chromium acetate does not exhibit any magnetic properties and is considered to be diamagnetic.
The diamagnetic behavior of chromium acetate can be explained by the principles of quantum mechanics and the orbital configuration of the atoms in the molecule.
Learn more about the diamagnetic nature , please visit the link given below;
https://brainly.com/question/29277857#
#SPJ11
2S+3O2=2SO3. If 4moles of sulfur reacts with 9. 5 moles of oxygen, how many moles of oxygen would remain after the reaction?
Answers
Answer: 3.5 moles
Explanation:
For every 2 moles of sulfur consumed, there are 3 moles of oxygen consumed.
This means that sulfur is the limiting reactant, meaning that 2(3)=6 moles of oxygen will be consumed.
Thus, 9.5-6=3.5 moles of oxygen will remain.
What is the correct expression for the equilibrium constant for the autoionization of water?ABCUnanswered
Answers
Kw is roughly equivalent to 1.0 x 10⁻¹⁴ at room temperature. The equilibrium constant for the autoionization of water, also known as the ion product constant, is expressed by the equation Kw = [H₃O⁺][OH⁻].
It represents the product of the concentrations of hydronium ions (H₃O⁺) and hydroxide ions (OH⁻) in a given solution of water at equilibrium. At room temperature, Kw is approximately equal to 1.0 x 10⁻¹⁴, which means that the product of [H₃O⁺] and [OH⁻] must always be equal to this value.
This equation is an expression of the law of mass action, which describes the equilibrium state of a reversible chemical reaction. The Kw value is important for calculating the pH of a solution and understanding the acidity and basicity of a substance.
To learn more about autoionization refer to:
brainly.com/question/28298480
#SPJ4
What is the correct expression for the equilibrium constant for the autoionization of water?
A
\(\frac{[\textrm{H}_3\textrm{O}^+][\textrm{OH}^-]}{[\textrm{H}_2\textrm{O}][\textrm{H}_2\textrm{O}]}\)
B
\(\frac{1}{[\textrm{H}_2\textrm{O}][\textrm{H}_2\textrm{O}]}\)
C
\([\textrm{H}_3\textrm{O}^+][\textrm{OH}^-]\)
Unanswered
What are the answers to these?

Answers
Answer:
1)physical change
Explanation:
2)chemical change
how many lines would be in the emission spectrum of hydrogen if the hydrogen atom had only 10 energy levels?
Answers
These are the energy emission spectrum of hydrogen.
What is energy emission spectrum?
When an atom or molecule changes from a high energy state to a lower energy state, electromagnetic radiation at a range of frequencies is released. This spectrum is known as the emission spectrum of a chemical element or chemical compound.
What is hydrogen ?
The most fundamental member of the chemical element family is hydrogen (H), a colorless, odorless, tasteless, combustible gaseous material.
There are generally 4 energy levels in a hydrogen spectrum. The lines formed is depends on the transitions occurs.
For n=6, there are 15 lines possibly formed. En to E(n-1) transitions are the possible transitions. It forms 5 series named Lyman, Balmer, Paschan, Brachet and Pfund series.
When n=6,
En to E(n-1) means 6 to 5, 5 to 4, 4 to 3, 3 to 2 and 2 to 1 therefore 5 lines formed.
En to E(n-2) means 6 to 4, 5 to 3, 4 to 2, 3 to 1 and therefore 4 lines formed.
En to E(n-3) means 6 to 3, 5 to 2, 4 to 1 and therefore 3 lines formed.
En to E(n-4) means 6 to 2, 5 to 1 and therefore 2 lines formed
En to E(n-5) means 6 to 1 and therefore 1 line is formed
Thus altogether 6 energy levels will give 15 lines.
Therefore, these are the energy emission spectrum of hydrogen.
Learn more about energy emission spectrum from the given link.
https://brainly.com/question/24213957
#SPJ4
HELP ILL GIVE BRAINLIEST
In the molecule above, the black circles are Carbon, the blue circles are Hydrogen and the red circles are Oxygen. Which formula below is correct for this molecule?
Question 4 options:
C4H4O4
C2H4O2
C2H2O4
C4H2O2

Answers
Answer:
C2H4O2
Explanation:
In this molecule two carbon molecule , four hydrogen molecules and two oxygen molecules are present . So C₂H₄O₂ is the correct molecular formula of this structure. Option B is correct.
What is molecular formula ?The molecular formula defines the number of atoms of each element in one molecule of a compound. It shows the actual number of each atom in a molecule.
An empirical formula represents the simplest whole-number ratio of various atoms present in a compound. The molecular formula shows the exact number of different types of atoms present in a molecule of a compound.
In this structure there are 2 carbon molecules, 4 hydrogen molecules and 2 oxygen molecules are present.
Hence , the option B is correct . The molecular formula of this structure is C₂H₄O₂.
To learn more about molecular formula refer the link below;
https://brainly.com/question/14425592
#SPJ2
what is matter made of?
Answers

Molar mass calculation for Cl^2
Answers
Answer:
Mass of Cl = 35.5
Mass of Cl2 => 35.5×2
=> 71 g/mol
hope that helps ✌
71
explain:The atomic mass of chlorine is equal to the molar mass of chlorine so the molar mass of chlorine is 35.5
=> molar mass for Cl^2: 35,5*2=71
how can i find wavelength in a wave?
Answers
Wavelength (L) is calculated using: L = gT²/2π, here g=9.8 m/s2 and T is wave period in seconds.
What is wavelength?Wavelength of a wave describes how long the wave is and the distance from the "crest" (top) of one wave to the crest of next wave is called wavelength. We can also measure from the "trough" (bottom) of one wave to trough of next wave and get the same value for the wavelength.
We measure wavelength in following ways:
Use photometer to measure the energy of wave.
Convert energy into joules (J).
Divide energy by Planck's constant, 6.626 x 10⁻³⁴, to get the frequency of wave.
Divide speed of light, ~300,000,000 m/s, by frequency to get wavelength.
To know more about wavelength, refer
https://brainly.com/question/10750459
#SPJ9
Which compound has a very large value of Ka in aqueous solution? a. H3PO4
b. NaCl
c. NH3
d. HNO3
e. KOH.
Answers
The compound which has a very large value of Ka in aqueous solution is HNO3.
Ka is an acid dissociation constant that measures the degree of ionization of an acid in a solution and tells how much of it will dissociate into its ions.
The larger the value of Ka, the greater the ionization and the stronger the acid.
Let's take a look at the dissociation equations and values of Ka of all the given compounds:
a. H3PO4:
H3PO4 ⇌ H+ + H2PO4- (Ka1 = 7.5 × 10^-3)
H2PO4- ⇌ H+ + HPO42- (Ka2 = 6.2 × 10^-8)
HPO42- ⇌ H+ + PO43- (Ka3 = 4.8 × 10^-13)
The successive values of Ka decrease greatly. Hence, H3PO4 is not the answer.
b. NaCl:
NaCl does not dissociate into ions in solution, so it is not an acid. Hence, it is not the answer.
c. NH3:
NH3 + H2O ⇌ NH4+ + OH- (Kb = 1.8 × 10^-5)
NH3 is not an acid; it is a weak base. Hence, it is not the answer.
d. HNO3:
HNO3 ⇌ H+ + NO3- (Ka = 24)
Nitric acid is a strong acid, and it has a very large value of Ka in aqueous solution. Hence, HNO3 is the answer.
e. KOH:
KOH ⇌ K+ + OH- (Kb = 1.0 × 10^-14)
KOH is not an acid; it is a strong base. Hence, it is not the answer.
learn more about aqueous solution on
https://brainly.com/question/32611537
#SPJ11
MARKING BRAINLIEST
answer

Answers
If you could repeat the lab and make it better, what would you do differently and why?
There are always ways that labs can be improved. Now that you are a veteran of this lab and have experience with the procedure, offer some advice to the next scientist about what you suggest and why. Your answer should be at least two to three sentences in length.
Answers
Hello, 3Coli Here!
Your Answer is Here:
I would recheck, redo, and revise my answers, if necessary. Also, I can look at the lesson again, if I forgot something. So that's what I would do.
Hopefully, this helps!
Ask your question below!
Answer:
I will just revise and check my answer, or I can redo the lesson.
Explanation:
Have a good day! :D
28.5 g of iron is added to a graduated cylinder containing 45.5 mL of water. The water level rises to 49.1 mL. The density of the iron is
Answers
Answer:
7.92 g/mL.
Explanation:
The following data were obtained from the question:
Mass of Iron = 28.5 g
Volume of water = 45.5 mL
Volume of water + Iron = 49.1 mL.
Density of Iron =.?
Next, we shall determine the volume of the iron. This can be obtained as illustrated below:
Volume of water = 45.5 mL
Volume of water + Iron = 49.1 mL.
Volume of iron =.?
Volume of iron = (Volume of water + Iron) – (volume of water)
Volume of iron = 49.1 – 45.5
Volume of iron = 3.6 mL
Finally, we shall determine the density of iron as follow:
Mass of Iron = 28.5 g
Volume of iron = 3.6 mL
Density of Iron =.?
Density = mass/volume
Density = 28.5/3.6
Density = 7.92 g/mL
Therefore, the density of the iron is 7.92 g/mL
Calculate the number of MOLES of oxygen
that will dissolve in a 50 L fish tank of water
at 25◦C if the partial pressure of oxygen is
0.21 atm. Henry’s constant for oxygen is 4.34
x 104
atm. Density of water = 1kg/L.
Answers
The number of moles of oxygen that will dissolve in a 50 L fish tank of water at 25°C with a partial pressure of 0.21 atm is approximately 0.0242 moles.
To calculate the number of moles of oxygen that will dissolve in the fish tank, we can use the formula:
Moles of O₂ = (Volume of water × Partial pressure of O₂ × Density of water) / Henry's constant
Given the volume of water (V) is 50 L, partial pressure of oxygen (P) is 0.21 atm, density of water (ρ) is 1 kg/L, and Henry's constant (K) for oxygen is 4.34 × 10⁴ atm, we can plug these values into the formula:
Moles of O₂ = (50 L × 0.21 atm × 1 kg/L) / (4.34 × 10⁴ atm)
Moles of O₂ = (10.5 kg) / (4.34 × 10⁴ atm)
Moles of O₂ ≈ 0.0242 moles
Therefore, approximately 0.0242 moles of oxygen will dissolve in a 50 L fish tank of water at 25°C if the partial pressure of oxygen is 0.21 atm.
Know more about Henry's Law Constant here:
https://brainly.com/question/30636760
#SPJ11
A student decreases the temperature of a 494 cm3 balloon from 455 k to 245 k.
assuming constant pressure, what should the new volume of the balloon be?
round your answer to one decimal place.
Answers
The new volume V₂ of the balloon is 266 cm³.
Calculation:The experimental gas law known as Charles' law describes how gases tend to expand when heated. The law of volumes is another name for it.
According to Charles's law,
V₁/T₁ = V₂/T₂
In question it has been given,
V = Volume
T = Temperature
V1 = 494cm3
T1=455k
T2=245k
V2 ( New Volume of Balloon)=?
Charles's law formula is : = V₁/T₁ = V₂/T₂
Putting values in Formula of charles's law
V₁/T₁ = V₂/T₂
= 494 / 455 = V₂/ 245
Cross Multiply each other,
V₂×455K= 494 cm³×245K
V₂ =494cm³×245K÷455K
V₂ = 266 cm³
Hence, the new volume of the balloon is 266 cm³
To know more about Charles' law :
https://brainly.com/question/16927784
#SPJ4
Q3. What happens when a copper atom becomes a copper(ll) ion? A) It is oxidized by losing two electrons B) It is oxidized by gaining two electrons C) It is reduced by gaining two electrons D) It is reduced by losing two electrons
Answers
Answer:
It reduces by ganing two electrons.
Select the change in matter that DOES NOT belong:
dissolving sugar
burning wood
breaking a bottle
melting wax
Answers
Answer:
Burning wood
Explanation:
Burning wood is a chemical change. Dissolving sugar is a physical change because it is still sugar, just dissolved. A broken bottle a physical change because it is just broken. Melting wax is a physical change because it is still wax, just melted. Burnt wood is a chemical change because it is now ash
Answer:
breaking a bottle
Explanation:
can there be 4 electrons in the first energy level
Answers
1. A group of chemistry students wants to find out if hydrochloric acid (HCl) is stronger than sulfuric acid
(H₂SO4) by observing how they react with baking soda. They plan to measure out equal amounts of
baking soda and place them into two identical test tubes. They will then add three drops of each acid
and use a stopwatch measure how long the reaction lasts in seconds.What is the independent variable, dependent variable, and hypothesis?
Answers
Hydrochloric acid is a stronger acid than sulphuric acid because the \(pK_a\)value of HCl is smaller than H₂SO₄
Why is hydrochloric acid stronger than sulphuric acid?Acids are substances that contain hydrogen and can donate H⁺ ions to another substance.
Hydrochloric acid and sulphuric acid both are stronger acids as compared to other acids
HCl dissolves in an aqueous solution to give out H⁺ and Cl⁻ and is completely dissociated.
H₂SO₄ dissolves in an aqueous solution to give out H₃O⁺ and HSO₄⁻ but the ions are partially dissociated
However, HCl is a stronger acid than H₂SO₄ because of the difference between the pKa values of both acids.
The \(pK_a\) value of HCl is -6.3 and the \(pK_a\) of H₂SO₄ is -2.8.
The smaller the \(pK_a\) value, the stronger the acid.
When HCl reacts with baking soda it gives out common salt, water, and carbon dioxide as gas. The reaction is exothermic
\(NaHCO_3 + 2HCl \rightarrow NaCl +H_2O + CO_2\)
Also, when H₂SO₄ reacts with baking soda it gives out sodium sulfate, water, and carbon dioxide as gas. The reaction is exothermic
\(2NaHCO_3+H_2SO_4 \rightarrow Na_2SO_4 +2H_2O + 2CO_2\)
Hence, HCl is a stronger acid than H₂SO₄ because of its \(pK_a\) value.
Learn more about acid:
https://brainly.com/question/25148363
#SPJ9
Choose the three factors that most significantly influence the properties of nanoscale materials.
a. bulk material properties.
b. elemental compostion.
c. size.
d. shape.
e. quantum mechanical effects
Answers
b. elemental composition d. shape and e. quantum mechanical effects three factors significantly influence the properties of nanoscale materials.
Nanoscale material are the set of substances that have least one Critical dimension less than 100 nanometer. That have magnetic, electrical and optical properties. S and P blocks are there in the nanoscale materials. Nanoscale materials are mostly concentrated in the s and p blocks Nano elements. Nanoscale material have have structure with dimensions at the nanoscale.so it has size of 1-100nm.The shape of the nanomaterials are in various shapes like rod shape spherical shape ,fibers and cups. In the nanoscales quantum effects dominates the electrical optical properties of the system. They create new opportunity for manipulating the response of the system.
To learn more about Nanoscale materials please visit:
https://brainly.com/question/10280062
#SPJ4
1
N
3
5
6
7
| 8 9 10
Which is evidence that a chemical reaction has likely occurred?
a liquid slowly losing volume
the formation of a precipitate
boiling water releasing steam
a change in the shape of a solid

Answers
Answer:
The new substance will need more energy to form its chemical bonds than the old substance will release. ... More energy will be released from the old substance than the new substance will need to form its chemical bonds.
Explanation:
This is the answer I got. Hope it's really helpful
Answer:
"Which is evidence that a chemical reaction has likely occurred?"
The correct answer would be,
B. the formation of a precipitate
Explanation:
Got it right on my test, have a great day!
Which of the following atoms in their ground states ae expected to be diamagnetic? Select all that applya) Bab) Sic) Znd) Se
Answers
Answer
a) Ba
b) Si
c) Zn
Explanation
The presence of unpaired electrons in an atom orbitals indicates paramagnetic properties. When no unpaired electron is observed in an atom orbitals, such an atom possesses diamagnetic properties.
Therefore, atoms in their ground states that are expected to be diamagnetic in the options are:
a) Ba
b) Si
c) Zn
Solutions can be concentrated or diluted. A student needs to select a diluted solution. Select the correct one. a. 35 g of solute dissolved in 0.1 L of water .b. 2.0 g of solute dissolved in 1.0 L of water. c. 30 g of solute dissolved in 1.0 L of water
Answers
The correct option for a diluted solution would be (b) 2.0 g of solute dissolved in 1.0 L of water.
The correct choice for a diluted solution is option B, 2.0 g of solute dissolved in 1.0 L of water. The concentration of this solution can be calculated as:
Concentration = mass of solute/volume of solution
Substituting the values, we get:
Concentration = 2.0 g / 1.0 L = 2.0 g/L
This concentration is relatively low, indicating a diluted solution. Option A has a high concentration of 350 g/L, making it a concentrated solution. Option C has a concentration of 30 g/L, which is lower than option A but higher than option B, making it a moderately concentrated solution.
To learn more about Diluted solution click here
https://brainly.com/question/30956529
#SPJ11
If the brine contains 138 g of NaCl, how much Cl2 can be produced?
Answers
Answer:
167.5g
Explanation:
From the question, we know the mass of NaCl and the molar mass of NaCl, so we can calculate the number of moles of NaCl:
Moles = mass ÷ molar mass
The molar mass of NaCl can be calculated by adding the relative formula masses of the elements Na and Cl:
23.0 + 35.5 = 58.5
We can use the formula to find the number of moles of NaCl:
moles = 138 ÷ 58.5
= 2.35897...
Now, we need to find the mass of Cl2. Rearranging the formula of moles = mass ÷ molar mass
mass = moles × molar mass
Calculating the molar mass of Cl2 by adding the relative atomic masses:
35.5 + 35.5 = 71
Hence the mass is:
2.35897... × 71 = 167.5g (to 1dp)
The activation energy Ea of a reaction is Ea = 43.5Kj.mol. Estimate the variation in the rate k of the reaction when the temperature is raised from 300K to 310K.(R = 8.314 J.K.mol)
Answers
Answer
The variation in the rate k (k1/k2) is 0.5707 s^-1
Step-by-step explanation:
Given the following parameters
Activation energy = 43.5Kj.mol
T1 = 300K
T2 = 310K
R = 8.314 J.Kmol
\(K\text{ =A}e^{-\frac{Ea}{RT}}^{}^{}\)\(\begin{gathered} \text{where K = rate constant},\text{ Ea = Activation energy, R= gas constant and T = temperature} \\ \ln (k)\text{ = }\ln A\text{ - }\frac{Ea}{RT} \\ \text{ A is a constant} \\ \text{ if the reaction occurs at two temperature T1 and T2} \\ \ln k1\text{ = }\ln A\text{ - }\frac{Ea}{RT1}--------\text{ equation 1} \\ \ln k2\text{ = }\ln A\text{ - }\frac{Ea}{RT2}\text{ --------- EQUATION 2} \\ \text{ substracting equation 1 from 2},\text{ we have} \\ \ln (\frac{k1}{k2})\text{ = }-\frac{Ea}{RT1}\text{ + }\frac{Ea}{RT2} \\ \ln (\frac{k1}{k2})\text{ = }\frac{Ea}{R}(\frac{1}{T2}\text{ - }\frac{1}{T1}) \\ \\ \ln (\frac{k1}{k2})\text{ = }\frac{43500}{8.314}(\frac{1}{310}\text{ - }\frac{1}{300}) \\ \ln (\frac{k1}{k2})\text{ = 5232.139 }(0.00322\text{ - 0.003333)} \\ \ln (\frac{k1}{k2})\text{ = 5232.139 (}-\text{ 0.0001071)} \\ \ln (\frac{k1}{k2})\text{ = -0.560851} \\ \text{Take the exponential of both sides} \\ \frac{k1}{k2}\text{ = }e^{-0.560851} \\ \frac{k1}{k2\text{ }}=0.5707s^{-1} \end{gathered}\)Questions 1-10: Complete the Bohr
Mode for each following elements.

Answers
Answer:
proposed an early model of the atom as a central nucleus containing protons and neutrons being orbited by electrons in shells. As previously discussed, there is a connection between the number of protons in an element, the atomic number that distinguishes one element from another, and the number of electrons it has. In all electrically-neutral atoms, the number of electrons is the same as the number of protons. Each element, when electrically neutral, has a number of electrons equal to its atomic number.
THANKS
3
Explanation:
what is an evenly distributed uniform mixture called?
Answers
Answer:
It is called homoogeneous
Answer:
it's called a homogeneous mixture.
John walks 10 miles a day. How many meters does he walk in 5 days?
Answers
Answer:
He will burn 50 meters in 5 days
Explanation:
multiply 10 and 5.
Answer:
80450 meters
Explanation:
To go from miles to meters multiply by 1609, so do 1609 x 10. Next, take that number times 5 because that is the number of days.
- Let me know if you need further explanation.
HELPPPP!!!! SCIENCE!!!!
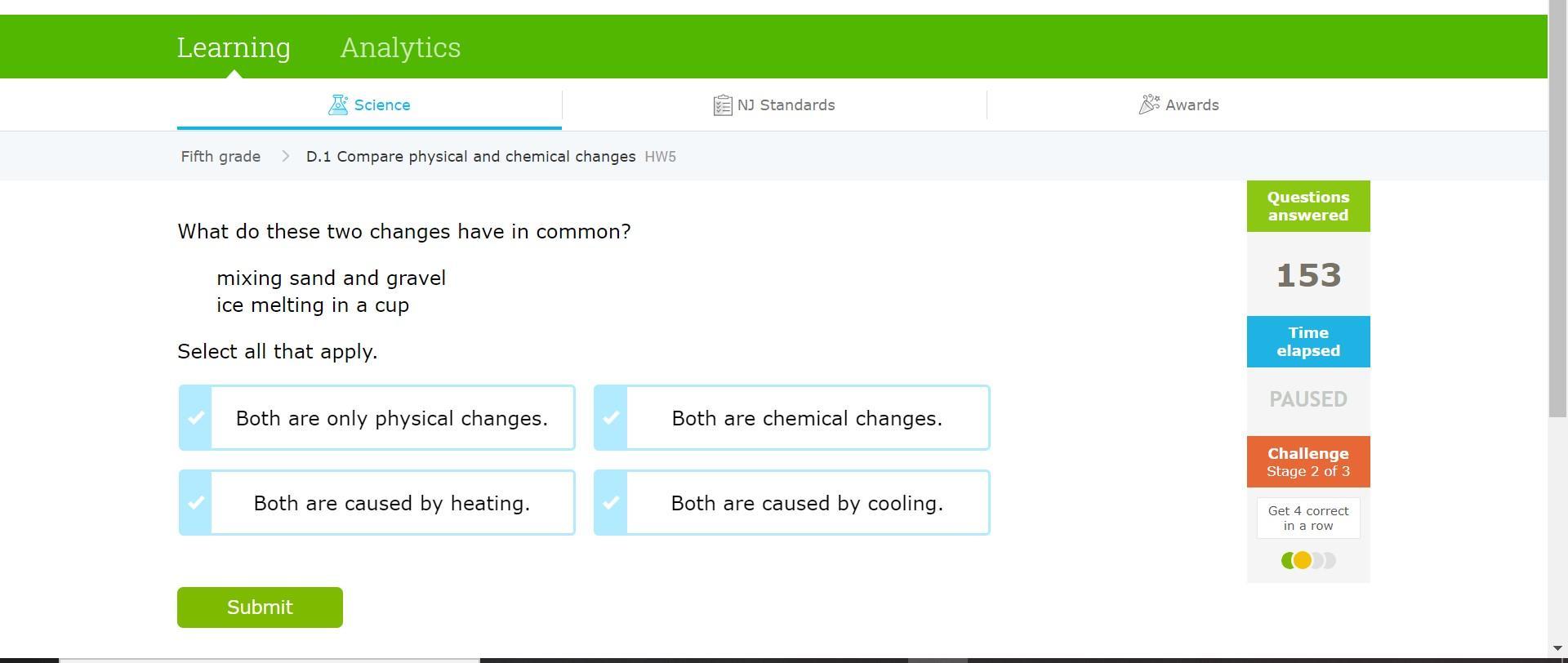
Answers
Answer:
Both are only physical changes