When titrating a NaF(aq) solution with HCl(aq), the equivalence point will occur at a pH value ______ .
a) above 7 because it is determined by NaF(aq)
b) below 7 because HCl(aq) is a strong acid
c) below 7 because it is determined by HF(aq)
d) at 7 because it is determined by H2O(l)
Answers
At the equivalence point, the NaF completely reacts with the HCl
The correct option that gives the equivalence point when titrating NaF solution with HCl(aq), occur at a pH value is option c);
c) Below 7 because it is determined by HF (aq)
Reason:
The ionic equation of the reaction taking place in the titration is presented as follows;
NaF + HCl → HF + NaClNa⁺ (aq) + F⁻ (aq) + H⁺ (aq) + Cl⁻(aq) → HF (aq)+ Na⁺(aq) + Cl⁻(aq)Therefore, by removing the spectator ions, we get;
F⁻(aq) + H⁺(aq) → HF(aq)Therefore;
The net ionic equation is the formation of hydrofluoric acid, HF, which is a
weak acid, therefore, the pH value will be below 7 because it is determined
by HF
Learn more about equivalence point here: titration
https://brainly.com/question/2496608
https://brainly.com/question/14819792
https://brainly.com/question/23502649
Related Questions
What element has the most similar property as fluorine
Answers
Answer:
Chlorine and Bromine are also halogens so they are similar to fluorine
1) A sample of an ideal gas has a volume of 2.27 L at 285 K and 1.10 atm. Calculate the pressure when the volume is 1.36 L and the temperature is 306 K.
Answers
A sample of an ideal gas has a volume of 2.27 L at 285 K and 1.10 atm. The pressure when the volume is 1.36 L and the temperature is 306 K is 1.77 atm.
What is an ideal gas equation ?
The ideal gas equation is represented as: PV = nRT. In this equation, P refers to the pressure of the ideal gas, V is the volume of the ideal gas, n is the total amount of ideal gas that is measured in terms of moles, R is the universal gas constant, and T is the temperature.
Given:
P1 = 1.10 atm
V1 = 2.27 L
T1 = 285 K
P2 = ?
V2 = 1.36 L
T2 = 306 K
By an ideal gas equation, we get
P2V2/T2 = P1V1/ T1
P2 = T2P1V1/ V2T1
= 306 × 1.10 × 2.27 / 1.36 × 285
= 764 / 387.6
= 1.77 atm
Thus, the pressure when the volume is 1.36 L and the temperature is 306 K is 1.77 atm.
To learn more about an ideal gas equation, follow the link;
https://brainly.com/question/28837405
#SPJ1
Determine the mass in each of the following
1) 54 molecules of CO2 (MM = 44.0 g/mol)
2) 3.65 x 1024 molecules of water (MM = 18.0 g/mol)
Answers
The mass of a substance with given number of molecules can be calculated by first finding the number of moles in the substance as follows:
No. of moles = no of molecules ÷ Avogadro's number
Moles of carbon dioxide = 54 ÷ 6.02 × 10²³ = 8.97 × 10²³ molesMoles of water = 3.65 x 10²⁴ ÷ 6.02 × 10²³ = 0.61 molesMass of these substances can be calculated by multiplying the number of moles by their molar mass as follows:
Mass of carbon dioxide = 8.97 × 10²³ moles × 44 g/mol = 3.95 × 10²⁵ grams. Mass of water = 0.61 moles × 18 g/mol = 10.98 gramsLearn more about mass at: https://brainly.com/question/21042927
#SPJ1
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
What does electric current measure?
O how well electrical energy moves in a circuit
O how quickly electrical energy moves in a circuit
O how frequently electrical energy moves in a circuit
O how much electrical energy moves in a circuit
Answers
Answer:
Explanation:
Electric current in a wire, where the charge carriers are electrons, is a measure of the quantity of charge passing any point of the wire per unit of time. ... Current is usually denoted by the symbol I. Ohm's law relates the current flowing through a conductor to the voltage V and resistance R; that is, V = IR.
Answer: O how well electrical energy moves in a circuit
Explanation: Electric current is a measure of the flow of charge, as, for example, charge flowing through a wire. Electric current in a wire, where the charge carriers are electrons, is a measure of the quantity of charge passing any point of the wire per unit of time.
Without SALT or SUGAR, does the water conduct electricity?
Answers
How to balance this equation using algebraic method Cu + HNO3 = Cu(NO3)2 + NO2 +H2O
Answers
1) Write the chemical equation.
\(Cu+HNO_3\rightarrow Cu(NO_3)_2+NO_2+H_2O\)Step 1: assign letters to stoichiometric coefficients.
\(ACu_{}+BHNO_3\rightarrow CCu(NO_3)_2+DNO_2+EH_2O_{}\)Step 2: list the elements involved in the reaction.
Cu: 1A = 1C
H: 1B = 2E
N: 1B = 2C + 1D
O: 3B = 6C + 2D + 1E
Step 3: assign an arbitrary number to one of the letters. Let's try C = 1.
Cu: 1A = 1C
1*A = 1*(1)
A = 1
Let's try E = 2.
H: 1B = 2E
1*B = 2*(2)
B = 4
Replace C and B in 1B = 2C + 1D
N: 1B = 2C + 1D
1*(4) = 2*(1) + 1D
4 = 2+ 1*D
D = 4-2 = 2
Step 4: replace the letter in the chemical equation and check.
\(Cu+4HNO_3\rightarrow Cu(NO_3)_2+2NO_2+2H_2O\).
In an experiment, a student dissolved 11.75 g of NaNO3 is an unknown mass of water initially at a temperature of 18.6 oC. After all the NaNO3 dissolved, the temperature of the solution was measured to be 12.5 oC. From this data, calculate the mass, in g, of water originally in the container before the NaNO3 was added.
111 was incorrect for me.

Answers
The mass of water initially present in the container is 11.16 g.
We are given the following:mass of NaNO3 = 11.75 ginitial temperature of water = 18.6 oC.final temperature of solution = 12.5 oCWe need to determine the mass of water initially present in the container. The key formula to use in this problem is the heat absorbed or lost formula, which is expressed as follows:q = m·c·ΔTwhere:q is the heat absorbed or lost in Joules (J)m is the mass of the substancec is the specific heat capacity of the substanceΔT is the change in temperature of the substance, expressed in degrees Celsius (oC)Firstly, let us determine the heat lost by the water. This heat is equal to the heat absorbed by the NaNO3, which can be expressed as follows:q NaNO3 = -q waterwhere the negative sign indicates that the water lost heat.q NaNO3 can be expressed as follows:q NaNO3 = m NaNO3 · c NaNO3 · ΔTwhere:m NaNO3 is the mass of NaNO3c NaNO3 is the specific heat capacity of NaNO3, which is 3.75 J/g·KΔT is the change in temperature of the solution, which is equal to (12.5 - 18.6) oC = -6.1 oC.Substituting the given values gives:q NaNO3 = (11.75 g) · (3.75 J/g·K) · (-6.1 oC)q NaNO3 = -257.49 J.Now we can determine the mass of water that was initially present in the container. The heat lost by the water is equal to the heat gained by NaNO3.
This can be expressed as follows:q water = -q NaNO3.Rearranging and substituting the given values gives:m water · c water · ΔT = -q NaNO3m water = (-q NaNO3) / (c water · ΔT) where:c water is the specific heat capacity of water, which is 4.184 J/g·KΔT is the change in temperature of the water, which is equal to (12.5 - 18.6) oC = -6.1 oC.Substituting the given values gives:m water = (-257.49 J) / [(4.184 J/g·K) · (-6.1 oC)]m water = 11.16 g.
for such more questions on mass
https://brainly.com/question/24191825
#SPJ8
select the common word on the outside of a shipping container that will alert you to the fact that the material inside is hazardous
Answers
The common word on the outside of a shipping container that will alert you to the fact that the material inside is hazardous is caution.
What are hazardous statements?Hazardous statements are those words or phrases that are labelled against chemicals which shows the degree of how dangerous the chemical are.
The common words that are used as hazardous statements which shows that a chemical is dangerous include the following:
Danger,Caution andWarning.Therefore, the common word on the outside of a shipping container that will alert you to the fact that the material inside is hazardous is caution.
Learn more about hazard statements here:
https://brainly.com/question/2875871
#SPJ1
2C2H6 + 7O2 —> 4CO2 + 6H2O how many grams of oxygen react in order to produce 7.2 moles of carbon dioxide
Answers
Answer:
403.2 grams of oxygen
Explanation:
According to the balanced chemical equation, 2C2H6 + 7O2 —> 4CO2 + 6H2O, 4 moles of carbon dioxide (CO2) are produced for every 7 moles of oxygen (O2) that react. Therefore, if 7.2 moles of carbon dioxide are produced, the number of moles of oxygen that react is (7.2 moles CO2) * (7 moles O2 / 4 moles CO2) = 12.6 moles O2.
Since the molar mass of oxygen is approximately 32 g/mol, the mass of oxygen that reacts is (12.6 moles O2) * (32 g/mol) = 403.2 g. Therefore, 403.2 grams of oxygen react in order to produce 7.2 moles of carbon dioxide.
Explain the relationship between atoms, elements, compounds, & mixtures.
PLEASE DONT JUST TAKE THE POINTS!!!
Answers
Answer:
**TAKES POINTS** lol just kidding!! heres your answer: basically all compounds and elements are composed of atoms :)
Explanation:
all elements, and compounds, all matter in point of fact, are composed of atoms. In a compound, there are 2 or more different types of atoms present that are chemically bound In an element there is only the one type of atom
what is the reaction?
Answers
Answer:
A chemical reaction is a process in which one or more substances, also called reactants, are converted to one or more different substances, known as products. ... A chemical reaction rearranges the constituent atoms of the reactants to create different substances as products.
Explanation:
Hopefully this is what you needed
Suppose a student repeats Experiment 1 using strontium instead of magnesium. The student adds 4.93 g of strontium to a crucible, heats the crucible and its contents for several minutes over a Bunsen burner, and records the final mass of the crucible and its contents.
Write the balanced chemical equation for this reaction. Include physical states.
balanced equation:
What mass of product is expected to form in this reaction? Assume all of the strontium reacts.
mass of product:
Answers
The balanced chemical equation for the reaction between strontium and oxygen can be written as follows: 2 Sr (s) + \(O_2\)(g) → 2 SrO (s).
In this equation, solid strontium (Sr) reacts with gaseous oxygen (\(O_2\)) to produce solid strontium oxide (SrO).
To determine the mass of product expected to form in this reaction, we need to consider the molar ratio between strontium and strontium oxide. From the balanced equation, we can see that 2 moles of strontium react to produce 2 moles of strontium oxide.
The molar mass of strontium (Sr) is 87.62 g/mol, and the molar mass of strontium oxide (SrO) is 119.62 g/mol. Since the molar ratio is 1:1 between strontium and strontium oxide, the mass of strontium oxide formed will be equal to the mass of strontium used.
In this case, the student added 4.93 g of strontium to the crucible. Therefore, the expected mass of strontium oxide formed will also be 4.93 g.
It's important to note that this calculation assumes that the reaction proceeds to completion, meaning that all of the strontium reacts with oxygen. In actual laboratory conditions, the yield of the reaction may be less than 100% due to factors such as incomplete reaction, side reactions, or product loss.
For more such questsion on balanced chemical equation visit:
https://brainly.com/question/11904811
#SPJ8
Given the following data. (i) Ca(s) + 2C(grafite) -> Cacis) X Ca(s) + ⅐0›(g) -> Cao(s) (iit) CaO(s) + H›O(I) -> Ca(OH)(ag) (iv) CHi(g) + 5/20,(8) -> 2C0,(g) + H,0(1) X* (v) C(grafite) + 02(g) -> CO›(g) [4 marks] AH = -62.8 kJ AH = -635.5 kJ AH = -653.1 kJ AH= -1300.0 kJ AH = -393.5 kJ / Calculate AH for the following reaction by using Hess's law and manipulating the given reactions: CaC(s) + H,O(I) - Ca(OH),(ag) + GHa(g) AH = ?
Answers
The enthalpy change (ΔH) for the reaction CaC(s) + H2O(I) → Ca(OH)(ag) + CH4(g) is -3617.6 kJ.
To calculate ΔH for the reaction CaC(s) + H2O(l) → Ca(OH)2(ag) + CH4(g), we can use Hess's law, which states that the enthalpy change of a reaction is independent of the pathway taken and depends only on the initial and final states.
We can manipulate the given reactions to obtain the desired reaction:
(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X (unknown value)
(ii) Ca(s) + 1/2O2(g) → CaO(s) ΔH = -635.5 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
Now, let's manipulate these equations to cancel out the common reactants and products and obtain the desired reaction:
(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X
(ii) Ca(s) + 1/2O2(g) → CaO(s) ΔH = -635.5 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
Now, let's sum up the equations to obtain the desired reaction:(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X
(ii) 2Ca(s) + O2(g) → 2CaO(s) ΔH = -1271 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
By adding equations (ii), (iii), (iv), and (v), we can cancel out CaO(s), H2O(l), and O2(g):
2Ca(s) + 2C(graphite) + CH4(g) → 2Ca(OH)2(ag) + CO(g) ΔH = X -1271 -653.1 -1300.0 -393.5
2Ca(s) + 2C(graphite) + CH4(g) → 2Ca(OH)2(ag) + CO(g) ΔH = X -3617.6 kJ
For more such questions on enthalpy visit:
https://brainly.com/question/14047927
#SPJ8
What is the energy of a photon of red light having a frequency of 3.08 x 10^14 HZ?
Answers
Answer:
2.04 x 10⁻¹⁹J
Explanation:
Given parameters:
Frequency = 3.08 x 10¹⁴Hz
Unknown:
The energy of the photon = ?
Solution:
The energy of a photon is given as;
E = hf
Where h is the planck's constant
f is the frequency
Insert the parameters and solve;
E = 6.63 x 10⁻³⁴ x 3.08 x 10¹⁴
E = 20.4 x 10⁻³⁴⁺¹⁴
E = 20.4 x 10⁻²⁰ = 2.04 x 10⁻¹⁹J
Answer:
2.97*10-19 j
Explanation:
E=Energy of photon
H=Planck constant=6.626*10-34
V=Frequency of photon
we can plug what we already know E=(6.626*10-34)(4.48*10 14)
if a=4 and b=3
What is the value of a²b²+b=
Answers
Answer:
hen ,b=4-a. From. Equation (2). Then b= 3. Now,,(a)square+(b)square=1square+3square. =1+9. =10. therefore the value of a square plus bsquare is 10
Explanation:
how many significant figures are contained in 38.7g, 2x10^18m, 3486002kg, 9.74150x10^4j, 0.0613cm^3,17.0kg, and 0.014g/mL
Answers
The number of significant figures contained in the following values are as follows:
38.7g - 32 x 10¹⁸m - 23486002kg - 79.74150 x 10⁴J - 60.0613cm³ - 317.0kg - 30.014g/mL - 2What is significant figures?Significant figures are digits that is meaningful with respect to the precision of a measurement.
Significant figures are digits that is nonzero, followed by a nonzero digit, or (for trailing zeroes) justified by the precision of the derivation or measurement.
According to this question, the number of significant figures contained in the following values are as follows:
38.7g - 32 x 10¹⁸m - 23486002kg - 79.74150 x 10⁴J - 60.0613cm³ - 317.0kg - 30.014g/mL - 2Learn more about significant figures at: https://brainly.com/question/14359464
#SPJ1
Balance the redox reaction by inserting the appropriate coefficients.
redox reaction:
H^{+} + ClO_{2}^{-} + I^{-} -> Cl^{-} + H_{2}O + I_{2}
H++ClO−2+I−⟶Cl−+H2O+I2
Answers
The redox reactions is balanced as by adding coefficients as follows:2 H+ +ClO²⁻+I⁻⟶2 Cl⁻+H₂O+I₂.
Redox reactions comprise of two parts a reduced part and an oxidized part, which occur simultaneously . The part which is reduced gain electrons and hence there is a increase in oxidation state of the species.
While, the part which is oxidized looses electrons and hence there is a decrease in oxidation state of the species.During redox reactions, there is no net change in the number of electrons . Electrons which are given off in oxidation are used up in reduction.These too are balanced by adding coefficients.
Learn more about redox reactions,here:
https://brainly.com/question/13293425
#SPJ1
When chlorobenzene reacts with Mg in ether followed by CO2 and neutralization with dilute HCl, __________ will be formed.
a. p-chlorobenzoic acid
b. p-deuterobenzoic acid
c. benzoic acid
d. benzene
e. No correct response
Answers
Answer:
c. benzoic acid
Explanation:
The given reaction is an example of a Grignard reaction:
When chlorobenzene (C₆H₅Cl) reacts with Mg in ether, an intermediate is formed (C₆H₅MgCl).
Said intermediate then reacts with CO₂ producing a benzoic acid salt (C₆H₅CO₂X), this salt is then neutralized with dilute HCl producing benzoic acid (C₆H₅CO₂H).
A chemical formula includes the symbols of the elements in the
compound and the subscripts that indicate*
how many atoms or ions of each type are combined in the simplest unit
the formula mass
the number of moles in each element
the charges of the elements or ions

Answers
Answer:
it indicates, how many atoms or ions of each type are combined in the simplest unit.
Please help. Thank you so much

Answers
Enthalpy change (H) and entropy change (S) are 11.7 103 Jmol-1 and 105 Jmol-1K-1, respectively, for a reaction at 25 °C.
What is the change in the free basic energy at 25 °C?The absolute entropies of a reactants and their products are S°(N2H4) (= half of this period J/(mol•K), S°(N2) = 191.6 J/(mol•K), or S°(H2) = 130.7 J/(mol•K) at 25°C, where the standard enthalpy change (H°) is 50.6 kJ/mol.
What is the calomel electrode's reduction potential at 25 C?E0 is known as 0.268 V at standard potential at 25°C, despite a slight variation in the computed value above. Similar to a silver-silver chloride electrode, the electrode potential is dependent on the chloride ion concentration.
To know more about entropy visit:
https://brainly.com/question/24278877
#SPJ1
Which of the following is a physical change?

Answers
How many milliliters of 0.500 M HBr would be required to react with 40.0 mL of 0.300 M Ca(OH)2?
Answers
Answer:
48dm³
Explanation:
Given reaction:
Ca(OH)₂ + 2HBr → CaBr₂ + 2H₂O
Parameters:
Concentration of HBr = 0.5M
Volume of Ca(OH)₂ = 40mL
Concentration of Ca(OH)₂ = 0.3M
Solution:
To solve this problem, we are going to use the mole concept. We solve from the known specie to the unknown.
We first find the number of moles of the known specie which is the Ca(OH)₂ ;
number of moles = concentration x volume
number of moles = 0.3 x 40 x 10⁻³ = 0.012moles
From the reaction equation;
1 mole of Ca(OH)₂ requires 2 moles HBr
0.012 moles of Ca(OH)₂ will require 0.012 x 2 = 0.024moles of HBr
Now,
To find the volume of HBr;
Volume = \(\frac{number of moles }{concentration}\)
Volume = \(\frac{0.024}{0.5}\) = 0.048dm³
In mL;
Volume 0.048 x 1000 = 48dm³
An unknown radioactive substance has a half-life of 3.20 hours.If 25.3 g
of the substance is currently present, what mass A0
was present 8.00 hours ago?
Express your answer with the appropriate units.
Answers
Therefore, mass A0 that was present 8.00 hours ago is 143.1g
Mass calculation.
The half life of the substance is 3.20 which means the substance will be halved at 3.20 hours.
At =AO*(1/2)(t/3.20)
We were given 25.3 g so lets substitute the value.
25.3 =AO*(1/2)(t/3.20)
25.3=AO*(1/2)(8/3.20)
25.3=AO*0.1768
AO= 25.3/0.1768
AO=143.1g
Therefore, mass A0 that was present 8.00 hours ago is 143.1g
Learn more about mass below.
https://brainly.com/question/22816432
#SPJ1
A sample of krypton gas at a pressure of 1.10 atm and a temperature of 22.6 °C, occupies a volume of 694 mL. If the gas is allowed to expand at constant temperature until its pressure is 0.765 atm, the volume of the gas sample will be
mL.

Answers
Considering the Boyle's law, the volume of the gas sample will be 997.908 mL.
Boyle's lawBoyle's law states that the pressure of a gas in a closed container is inversely proportional to the volume of the container, when the temperature is constant: if the pressure increases, the volume decreases while if the pressure decreases, the volume increases.
Mathematically, this law expresses that if the amount of gas and the temperature remain constant, the product of the pressure by the volume always has the same value:
P×V= k
where P is the pressure, V is the volume, and k is a constant.
Analyzing an initial state 1 and a final state 2, it is fulfilled:
P₁×V₁= P₂×V₂
New volume in this caseIn this case, you know:
P₁= 1.10 atm
V₁= 694 mL
P₂= 0.765 atm
V₂= ?
Replacing in Boyle's law:
1.10 atm× 694 mL= 0.765 atm×V₂
(1.10 atm× 694 mL)÷ 0.765 atm= V₂
997.908 mL= V₂
Finally, the new volume will be 997.908 mL.
Learn more about Boyle's law:
brainly.com/question/4147359
#SPJ1
Does anyone know the answer to this question

Answers
Answer:
A
Explanation:
If Hydrogen is H₂ There will be two silver
and is Carbon is C There will only be one gray
and if Oxygen is O₃ There will be three red
Select the correct structure that
corresponds to the name.
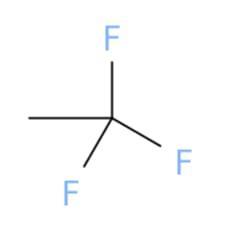
1,1,1-trifluoroethane

Answers
The correct chemical structure that corresponds to 1,1,1-trifluoroethane is (a).
What is 1,1,1-trifluoroethane?
A chemical structure is a spatial arrangement of atoms in a molecule. It determines the molecular geometry and when necessary the electronic chemistry as well .1,1,1-Trifluoroethane or simply known as trifluoroethane is Hydrofluorocarbon (HFC) compound that is colourless and highly inflammable gas with ether like odour. One method of preparation of 1,1,1-Trifluoroethane is by fluorination of 1-chloro-1,1-difluoroethane in the presence of hydrofluoric acid. The chemical formula for 1,1,1-Trifluoroethane is \(C__{2} } H_{3} F_{3}\). The high stability of it's chemical structure because of being heavier than air makes it a greenhouse gas with high infrared absorbent power. It can be used as a propellant or refrigerant and in cleaning of electrical equipments.
Learn more about 1,1,1-trifluoroethane here:
https://brainly.com/question/1390779
#SPJ1
Boiling point elevation means that:
A. it will take more energy for the particles of the solution to overcome the vapor pressure.
B. it will take more energy for the particles of the solution to overcome the atmospheric pressure.
C. the boiling point is lower because of the solute particles that are removed from the solution.
D. the boiling point is higher because of the solute particles that are removed from the solution.
Answers
Answer:
B. it will take more energy for the particles of the solution to overcome the atmospheric pressure.
Explanation:
i checked the slideshow from educere lol (slide 8 colligative properties lesson)
Boiling point elevation means it will take more energy for the particles of the solution to overcome the atmospheric pressure. Hence, option B is correct.
What is boiling point elevation?The temperature at which a liquid's vapor pressure equals that of the atmosphere is known as the boiling point. A nonvolatile solute solution has a boiling point that is greater than the pure solvent.
Pure water has a boiling point of 100 degrees Celsius, but eating a solute like salt can raise the boiling point. Only the solute's mole fraction determines the boiling point elevation. The molarity of the solution directly relates to the change in boiling point.
With an increase in external pressure, the boiling point rises. High boiling points are the property of liquids with stronger intermolecular forces.
Elevated boiling points indicate that more energy will be required for solution particles to overcome air pressure. As a result, choice B is accurate.
Learn more about Boiling point elevation. Here:
https://brainly.com/question/12951501
#SPJ5
How many grams of nacl are required to make 250.0 ml of a 3.000 m solution?
Answers
Answer:
43.83 grams of NaCl are required to make 250 ml of a 3M solution.
Explanation:
As given in the question,
Molarity of solution= 3M
Volume of solution= 250ml
Weight of solute Nacl=?
The formula for molarity is,
Number of moles=0.75 moles
According to the formula,
As we know, the molecular mass of NaCl is 58.44
Hence,
If 2.00 moles of H₂ and 1.55 moles of O₂ react how many moles of H₂O can be produced in the reaction below?
2 H₂(g) + O₂(g) → 2 H₂O(g)
Answers
Answer:
2 mol H₂O
Explanation:
With the reaction,
2H₂(g) + O₂(g) → 2 H₂O(g)1.55 moles of O₂ would react completely with ( 2*1.55 ) 3.1 moles of H₂. There are not as many moles of H₂, thus H₂ is the limiting reactant.
Now we calculate the moles of H₂O produced, starting from the moles of limiting reactant:
2.00 mol H₂ * \(\frac{2molH_2O}{2mol H_2}\) = 2 mol H₂OThe number of moles of H₂O that could be produced in the reaction is 2.00 moles
From the question,
We are to determine the number of moles of H₂O that could be produced.
The given balanced chemical equation is
2 H₂(g) + O₂(g) → 2 H₂O(g)
This means,
2 moles of H₂(g) reacts with 1 mole of O₂(g) to produce 2 moles of H₂O
From the given information
Number of moles of H₂ present = 2.00 moles
and
Number of moles of O₂ present = 1.55 moles
Since,
2 moles of H₂(g) reacts with 1 mole of O₂(g) to produce 2 moles of H₂O
Then,
The 2.00 moles of H₂(g) will react with 1.00 mole of O₂(g) to produce 2.00 moles of H₂O
∴ 2.00 moles of H₂O will be produced during the reaction
(NOTE: Only 1.00 mole of the O₂(g) will react, meaning O₂(g) is the excess reactant)
Hence, the number of moles of H₂O that could be produced in the reaction is 2.00 moles
Learn more here: https://brainly.com/question/1553639