Answers
Answer:
sliding friction occurs
Answer:
Sliding friction is occurs
Related Questions
You make Kool- Aid. Unfortunately, you misread the directions and made 0.512 L at a concentration of 13.2 M. In order for it to taste good it needs to be only 2.00 M. How much water do you need to add to make taste good? Show your work.
Answers
To dilute the Kool-Aid solution to a concentration of 2.00 M, we need to calculate the volume of water (Vw) that needs to be added.
The initial concentration of Kool-Aid solution is 13.2 M, and the initial volume is 0.512 L. Let's assume that Vw is the volume of water we need to add to dilute the solution to a concentration of 2.00 M.
Since the total volume after dilution will be the sum of the initial volume of Kool-Aid solution and the volume of water added, we can set up the following equation based on the dilution formula:
C1V1 = C2V2
where
C1 = initial concentration of Kool-Aid solution = 13.2 M
V1 = initial volume of Kool-Aid solution = 0.512 L
C2 = final concentration of Kool-Aid solution = 2.00 M
V2 = final volume of Kool-Aid solution after adding water = 0.512 L + Vw
Substituting the given values into the equation, we get:
13.2 M x 0.512 L = 2.00 M x (0.512 L + Vw)
Simplifying the equation, we get:
6.7584 L·M = 1.024 L·M + 2.00 M·Vw
Subtracting 1.024 L·M from both sides, we get:
5.7344 L·M = 2.00 M·Vw
Dividing both sides by 2.00 M, we get:
Vw = 2.8672 L
Therefore, we need to add 2.8672 L of water to 0.512 L of 13.2 M Kool-Aid solution to dilute it to a concentration of 2.00 M.
Know more about Kool-Aid solution here:
https://brainly.com/question/27297342
#SPJ11
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
Help pleaseeeeeeeee just need 4 answers
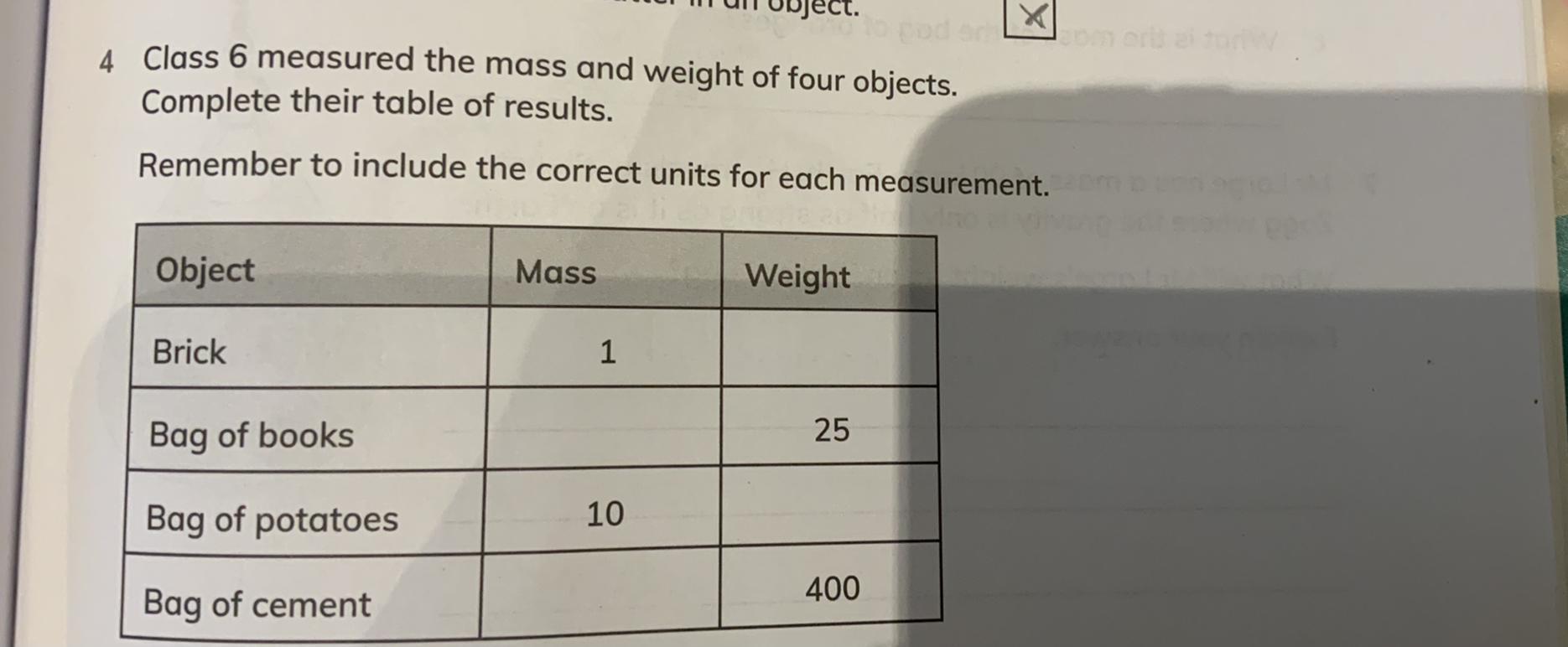
Answers
Answer:
Here, acceleration due to gravity(a) is assumed as 10m/s².We can also take it as 9.8m/s²
Explanation:\(Brick) Weight=m * a=1 * 10=10N\\Bag of books) Mass=f/a=25/10=2.5kg\\Bag of potatoes) Weight=m * a=10 * 10=100N\\Bag of cement) Mass=f/a=400/10=40kg\)
Name of this product

Answers
Answer:
Explanation:
ethyl 3-methylbenzoate
the weak ionization constant (Ka) for HNO2 is equal to:

Answers
Answer:
the answer is A
Explanation:
The weak ionization constant (Ka) for HNO₂ is:
\(\displaystyle K_a = \frac{[H^+][NO_2^-]}{HNO_2}\)
What is the ionization constant?Acid-ionization constant Ka can be described as a quantitative measure of the strength of an acid in solution. It can be represented as the equilibrium constant for a chemical reaction:
\({\displaystyle {\ce {HA \longrightarrow A^- + H^+}}}\)
The chemical species HA can dissociate into A⁻ the conjugate base of the acid and a hydrogen ion, H⁺. In equilibrium, when the concentrations will not change over time, because both forward and backward reactions have the same rate.
The ionization constant can be described as the ratio of products and reactants raised to stoichiometric powers.
The dissociation constant is defined as:
\({\displaystyle K_{\text{a}}=\mathrm {\frac {[A^{-}][H^{+}]}{[HA]}} }\)
Given the dissociation of the HNO₂ as follows:
HNO₂ ⇄ H⁺ + NO₂⁻
The weak ionization constant (Ka) for HNO₂ is equal to:
\(\displaystyle K_a = \frac{[H^+][NO_2^-]}{[HNO_2]}\)
Therefore, option A is correct.
Learn more about ionization constant, here:
https://brainly.com/question/13794673
#SPJ2
A speed boat, at full throttle, can go 65.0 miles in 1.5 hours. What is the average speed of the boat?
Answers
The average speed = 43.33 miles per hours(mph)
Further explanationGiven
distance = 65 miles
time = 1.5 hours
Required
The average speed
Solution
The average speed : distance traveled divided by time taken or total distance divided by total elapsed time
Can be formulated :
avg speed = distance : time
avg speed = d : t
Input the value :
avg speed = 65 miles : 1,5 hours
avg speed = 43.33 miles per hours(mph)
I REALLY NEED HELP can someone help me please I’ll mark you brainliest! :)

Answers
Answer:
A
Explanation:
The heat of fusion of water is 79.9 cal/g. If a 5 g piece of ice melts in 100 g of water at 30.0 °C in an insulated bottle, what is the final temperature of the water?
Answers
Thus, the heat of fusion of water is 79.9 cal/g. If a 5 g piece of ice melts in 100 g of water at 30.0 °C in an insulated bottle, the final temperature of the water is 35071 °C.
What is heat of fusion?Heat of fusion is defined as the amount of heat required to turn 1g of solid into a liquid without causing a temperature change. The term "heat of fusion" refers to the energy needed to melt a specific quantity of a solid at its melting point temperature.
To calculate final temperature
\(\rm q = mc \Delta\ T\)
Δ T = T(initial) - T(final)
T(final)= m × c × q - T(initial)
T(final)= 79.9 x 4.184 x 105 - 30.0
T(final)= 35071.6 °C
Thus, the heat of fusion of water is 79.9 cal/g. If a 5 g piece of ice melts in 100 g of water at 30.0 °C in an insulated bottle, the final temperature of the water is 35071 °C.
To learn more about heat of fusion, refer to the link below:
https://brainly.com/question/14053504
#SPJ1
Which of the following is true about the Lewis structure of NF3? Really need help

Answers
Answer:
All of the above.
Explanation:
Hello there!
In this case, according to the definition of the Lewis structures, which are represented by the valence electrons, we first identify that the N atom has five valence electrons and each fluorine has seven valence electrons.
In such a way, we cans say that N is the central atom due to its lower electronegativity, the molecule has 7+7+7+5=26 valence electrons and the three F-N bonds are covalent, therefore the answer is all of the above.
Regards!
4 Fe(s) + 3 02(g)
--> 2 Fe₂O3(s)
1. What is the oxidation state of iron (Fe) in the reactant Fe(s)?
2. What is the oxidation state of oxygen (O) in the reactant O2(g)?
3. What is the oxidation state of iron (Fe) in the product Fe2O3(s)?
4. What is the oxidation state of oxygen (O) in the product Fe2O3(s)?
5. In this reaction, iron is... (oxidized or reduced?)
6. In this reaction, oxygen is... (oxidized or reduced?)
7. What was the oxidizing agent in this reaction: Fe(s) or O2(g)?
Answers
The oxidation number of reactant Fe is 0 while the oxidation number of iron in the product is +3
What s a redox reaction?The term redox reaction implies a reaction in which there is an increase in the oxidation number of a specie and the decrease in the oxidation number of another specie.
Now we have the answers as follows;
1) The oxidation number of reactant Fe is 0
2) The oxidation number of reactant oxygen is 0
3) The oxidation number of iron in the product is +3
4) The oxidation number of oxygen in the product is -2
5). Iron is oxidized in the reaction
6) Oxygen is reduced in the reaction
7) The oxidizing agent in this case is the oxygen atom
Learn ore about redox reaction:https://brainly.com/question/13293425
#SPJ1
The chemical on this list which is not a humectant is: O sorbitol O glycerol O lanolin O methyl O alcohol
Answers
Option (e) is correct. Alcohol is not a humectant because it binds to water and dehydrates the body.
Humectant is a substance which is used to keep things moist. A humectant attracts and retains the moisture in the air nearby via absorption. Sorbitol is a naturally occurring polyol. It is widely used in the food industry as a humectant. Sorbitol is a good humectants. Glycerol is a humectant which is most often derived naturally from vegetable oils. Humectants work to preserve other skincare ingredients and retain moisture by drawing water from the surface of your skin down into the deeper skin layers. Lanolin oil softens the skin and is a good humectant making it ideal for use in you skin care products. Methyl is an extremely effective humectant for both rinse off and leave on products. It is recommended for use in skin care products including lotions and creams.
To learn more about Humectant please visit:
https://brainly.com/question/947751
#SPJ4
Rank the following iron-carbon alloys and associated microstructures from the hardest to the softest: __________.
(a) 0.25 wt% C with coarse pearlite,
(b) 0.80 wt% C with spheroidite,
(c) 0 25 wt% C with spheroidite, and
(d) 0.80 wt% C with fine pearlite.
Justify this ranking.
Answers
Answer:
The Ranking from
Hardest to softest is as follows :
0.80 wt% C with fine pearlite.
0.80 wt% C with spheroidite
0.25 wt% C with coarse pearlite
0.25 wt% C with spheroidite
Explanation:
To find - Rank the following iron-carbon alloys and associated microstructures from the hardest to the softest. Justify this ranking.
Solution -
Ranking is as follows :
(d) 0.80 wt% C with fine pearlite.
(b) 0.80 wt% C with spheroidite
(a) 0.25 wt% C with coarse pearlite
(c) 0 25 wt% C with spheroidite
Justification -
For some wt% C,
Fine pearlite is stronger than spheroidite
and
Coarse pearlite is stronger than spheroidite.
Now,
Due to carbon content,
0.80 wt% C with spheroidite is stronger than 0.25 wt% C with coarse pearlite.
So,
The Ranking from
Hardest to softest is as follows :
0.80 wt% C with fine pearlite.
0.80 wt% C with spheroidite
0.25 wt% C with coarse pearlite
0.25 wt% C with spheroidite
8. Match the following:
1.Warp
2. Retting
3.Ginning
4.Weft
a. Removal of gunny matter from the stem of a flax or jute plant by bacterial action in stagnant water.
b. The length wise yarn in the loom.
c. The cross wise yarn in the loom. d.. Removal of seeds from cotton
Answers
Answer:
c
Explanation:
Chemistry (Chemical Bonding Test)
A triple covalent bond shares
electrons between atoms.
Select one:
O a. Two
O b. Three
O c. Four
O d. Flve
O e. Six
O f. Unable to tell
Answers
what are the elements in group 5 in the periodic table
Answers
Answer:
Vanadium (V), Niobium (Niobium), Tantalum (Ta), Dubnium (Db), Cerium (Ce), and Thorium (Th)
Explanation:
Groups go vertical (up and down) on the periodic table.
Periods go horizontal (left to right) on the period table.
A solution of KC2H3O2 is diluted from its original concentration of 2.3 M to a new concentration 2.1 M. If it’s new volume is 191.8 mL, what was the original volume of the concentration solution?
Answers
The original volume of the concentrated solution was 182.7 mL.
To solve this problem, we can use the formula for dilution:
C1V1 = C2V2
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
We are given that the initial concentration (C1) is 2.3 M, the final concentration (C2) is 2.1 M, and the final volume (V2) is 191.8 mL. We want to find the initial volume (V1).
Plugging in the values we know into the dilution formula, we get:
(2.3 M) V1 = (2.1 M) (191.8 mL)
Simplifying this expression, we can solve for V1:
V1 = (2.1 M) (191.8 mL) / (2.3 M)
V1 = 182.7 mL
It's important to note that the units of concentration and volume must be consistent in this formula. In this case, the concentrations are given in units of M (moles per liter), and the volumes are given in units of mL (milliliters).
For such more questions on concentrated
brainly.com/question/29661329
#SPJ11
In a covalent bond electron pairs are
Answers
Answer:
A covalent bond, also called a molecular bond, is a chemical bond that involves the sharing of electron pairs between atoms. These electron pairs are known as shared pairs or bonding pairs, and the stable balance of attractive and repulsive forces between atoms, when they share electrons, is known as covalent bonding.Explanation:have a good day :P
The bond angle in h2s is?

Answers
Answer:
90^0.
Explanation:
The bond angle in H2O is 105^0 and in H2S it is 90^0.
Problem PageQuestion Nitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this: (aq)(g)(g)(l) At a certain temperature, a chemist finds that a reaction vessel containing a mixture of nitric acid, nitrogen monoxide, nitrogen dioxide, and water at equilibrium has the following composition: compound amount Calculate the value of the equilibrium constant for this reaction. Round your answer to significant digits. Clears your work. Undoes your last action. Provides information about entering answers.
Answers
Answer:
3.4
Explanation:
Nitric acid and nitrogen monoxide react to form nitrogen dioxide and water, like this: 2 HNO₃(aq) + NO(g) ⇄ 3 NO₂(g) + H₂O(l)
At a certain temperature, a chemist finds that a 9.5L reaction vessel containing a mixture of nitric acid, nitrogen monoxide, nitrogen dioxide, and water at equilibrium has the following composition:
compound amount
HNO₃ 15.5g
NO 16.6g
NO₂ 22.5g
H₂O 189.0g
Calculate the value of the equilibrium constant Kc for this reaction. Round your answer to 2 significant digits.
Step 1: Write the balanced equation.
2 HNO₃(aq) + NO(g) ⇄ 3 NO₂(g) + H₂O(l)
Step 2: Calculate the molar concentration of the species at equilibrium
We will use the following expression.
M = mass of solute / molar mass of solute × liters of solution
[HNO₃] = 15.5g / 63.01 g/mol × 9.5 L = 0.026 M
[NO] = 16.6g / 30.01 g/mol × 9.5 L = 0.058 M
[NO₂] = 22.5g / 46.01 g/mol × 9.5 L = 0.051 M
We do not calculate the molarity of water because it is a pure liquid and will not be included in the equilibrium constant.
Step 3: Calculate the equilibrium constant (Kc)
Kc = [NO₂]³/[HNO₃]²×[NO]
Kc = 0.051³/0.026²×0.058
Kc = 3.4
The chemical equation below is unbalanced. CaS + AlC → A + CaC Balance this equation.
Answers
The balanced chemical equation is CaS + AlC → A + CaC
To balance the chemical equation CaS + AlC → A + CaC, we need to ensure that the same number of atoms of each element is present on both sides of the equation. Here's the step-by-step process to balance the equation:
Begin by counting the number of atoms of each element on both sides of the equation.
Left side (reactants):
Calcium (Ca): 1
Sulfur (S): 1
Aluminum (Al): 1
Carbon (C): 1
Right side (products):
A: 1
Calcium (Ca): 1
Carbon (C): 1
Sulfur (S): 0
Start by balancing the elements that appear in the fewest compounds. In this case, we can balance sulfur (S) first. Since there is only one sulfur atom on the left side and none on the right side, we need to add a coefficient of 1 in front of A on the right side to balance the sulfur.
CaS + AlC → 1A + CaC
Next, balance calcium (Ca) by adding a coefficient of 1 in front of CaS on the left side.
1CaS + AlC → 1A + CaC
Now, balance aluminum (Al) by adding a coefficient of 1 in front of AlC on the left side.
1CaS + 1AlC → 1A + CaC
Finally, balance carbon (C) by adding a coefficient of 1 in front of CaC on the right side.
1CaS + 1AlC → 1A + 1CaC
The balanced chemical equation is:
CaS + AlC → A + CaC
For more question on balanced chemical equation visit:
https://brainly.com/question/30196693
#SPJ8
What is the percent mass of 37.5 g NaCl dissolved in 137.5 g water?
Answers
Step 1
% by mass is defined as:
mass of solute ----- 100 g of solution
Solution = solute + solvent
Solute = NaCl
Solvent = Water (H2O)
----------------------------------------------
Step 2
Mass of solution = 37.5 g (NaCl) + 137.5 g (water) = 175 g
----------------------------------------------
Step 3
Procedure:
37.5 g NaCl ----- 175 g solution
X ----- 100 g solution
X = 100 g solution x 37.5 g NaCl/175 g solution = 21.4 g NaCl = 21.4 % by mass
Answer: % by mass = 21.4 %
17. Ethanol can undergo two different elimination reactions. Identify the atoms eliminated when ethanol undergoes elimination to form (a) ethene and (b) ethanal (acetaldehyde). What reagents would you use for the two reactions?
Answers
Dehydration is the kind of reaction that involves the loss of a water molecule from the substrate.
What is dehydration?The term dehydration is the kind of reaction that involves the loss of a water molecule from the substrate. in this case the substrate that participates in the reaction is ethanol.
In the formation of ethene(ethylene) as shown, the atoms that are eliminated are hydrogen and oxygen. Thus the elimination of water from ethanol is how we form ethene.
On the other hand, the removal of hydrogen and oxygen from ethanol gives ethanal. In the case of the formation of ethanal, the reaction occurs under oxidizing conditions.
Learn more about dehydration reaction:https://brainly.com/question/13448613
#SPJ1


Select the correct answer.
Why are metals good conductors of heat and electricity?
A.
anions rotate around the stationary electrons
B.
cations rotate around the stationary electrons
C.
free moving atoms in metals carry both heat and electric current
D.
free moving electrons in metals carry both heat and electric current
E.
free moving protons in metals carry both heat and electric current
Answers
Answer:
D
Explanation:
the correct answer is D
which of the following is NOT a characteristic of minerals
A. Occur in gaseous and liquid state
B. Formed by inorganic processes
C. naturally occurring
D. definite chemical composition
Answers
Answer:
A. Occur in gaseous and liquid state
Explanation:
The choice that is not a characteristic of minerals is that minerals occur in gaseous and liquid state.
All minerals are solid inorganic compounds.
A mineral is an inorganic compound that is formed naturally. They have a definite and specific chemical composition. Minerals are the building blocks of rocks. When minerals aggregates together, they form different rock types. There is no known mineral that is in fluid state. All minerals are solids. Examples are quartz, kaolinite, gypsum e.t.cWhat volume (mL) of concentrated solution of magnesium chloride (9.00 M) must be diluted to 350. mL to make a 2.75 M solution of magnesium chloride?
Answers
\(❤Answer \)
Volume of 106.9 mL from the concentrated solution should be taken and diluted to 350 mL.
⠀
⠀
Main I'd On Indian Brainly Is - HeartCrush
\(⚡ Explantion\)
We can use the formula.
c1v1 =c2v2
Where c1 is the concentration and v1 is volume of the concentrated solution.
c2 is the concentration
and v2 is the volume of the diluted solution to be prepared
9.00 M x V1 = 2.75 M x 350 mL
V1 = 106.9 mL
Volume of 106.9 mL from the concentrated solution should be taken and diluted to 350 mL.
how can we get propanal from acetone
Answers
To convert acetone (propanone) to propanal, you can use a two-step process involving reduction and oxidation reactions. Here's a general outline of the process:
1. Reduction of Acetone to Isopropanol:
First, you need to reduce acetone to isopropanol (2-propanol) using a reducing agent. Common reducing agents for this step include sodium borohydride (NaBH₄) or lithium aluminum hydride (LiAlH₄).
Reaction conditions:
- Acetone + NaBH₄ (or LiAlH₄) → Isopropanol
- Solvent: usually an alcohol (e.g., methanol or ethanol) or an ether (e.g., THF)
- Temperature: room temperature or slightly above
2. Oxidation of Isopropanol to Propanal:
Next, oxidize isopropanol to propanal using an appropriate oxidizing agent. A common oxidizing agent for this step is pyridinium chlorochromate (PCC), which selectively oxidizes primary alcohols to aldehydes without over-oxidizing to carboxylic acids.
Reaction conditions:
- Isopropanol + PCC → Propanal
- Solvent: an aprotic polar solvent (e.g., dichloromethane)
- Temperature: room temperature
- Avoid strong oxidizing agents like potassium permanganate (KMnO₄) or chromium trioxide (CrO₃) because they can over-oxidize the isopropanol to propionic acid.
Rupert had three substances. A brown substance was a liquid at room
temperature. He hit each of the other two with a hammer. A blue crystal
cracked but did not break. A silver substance flattened but did not crack.
Which two statements could be true?
A. The brown substance is ionic
B. The silver substance is ionic
C. The brown substance is molecular
D. The blue substance is ionic
Answers
Answer:
its C and D
C. The brown substance is molecular
D. The blue substance is ionic
Explanation:
did the test !
Two correct statements are B) The silver substance is ionic
C) The brown substance is molecular.
What kind of substance is silver?Silver is a chemical element with the symbol Ag and atomic wide variety 47. categorized as transition steel, Silver is stable at room temperature.
Which substance is molecular?It is a molecular substance, that's a substance with or more atoms, the smallest gadgets of remember joined together via a covalent bond. A covalent bond is a hyperlink created via the sharing of electrons that holds these atoms collectively.
Learn more about substances here: https://brainly.com/question/2901507
#SPJ2
A person is going for a walk in the evening. To make sure that she can be seen walking when it gets dark, she wears a glow stick around her neck. The glow stick can be used as a light source when she bends the stick. Although the stick is sealed, bending the stick allows two chemicals to come in contact and react with each other. During the chemical reaction, light is given off by the glow stick.
When the reaction stops, light is no longer given off. What happens to the mass of the glow stick after the chemical reaction occurs? Explain citing evidence from the prompt and identify the scientific law that supports your explanation.
(give me claim,evidence,reasoning)
Answers
From the law of conservation of mass, the mass of the glow stick does not change after the reaction.
Law of conservation of massFrom the law of conservation of mass we know that mass can neither be created nor destroyed but is converted from one form to another. Since the mass of the system must remain unchanged, It then follows that the mass of the glow stick must remian the same.
Therefore, the mass of the glow stick does not change after the reaction following the law of conservation of mass.
Learn more about conservation of mass: https://brainly.com/question/13383562
How might the puppy’s new environment affect its growth and development?
Answers
Answer:
New environments for puppies may make them excited, fearful, aggressive, or confused.
Explanation:
"When your dog first experiences a new location or environment, there's no way of knowing how they'll react. New sights, sounds, and smells could make them fearful, aggressive, or over-excited, but with the proper training and introduction, most dogs will quickly adapt and start taking every new location in stride." - Excerpt from *Puppy training textbook*
New environments are also a good thing in puppy growth, to teach them how to learn and adapt to new surroundings.
Hope this helps :)
how is hesses law used to calculate the enthalpy of a reaction
Answers
Answer:
Explanation:
Hess’s law derives directly from the law of conservation of energy, as well as its expression in the first law of thermodynamics. By Hess’s law, the net change in enthalpy of the overall reaction is equal to the sum of the changes in enthalpy for each intermediate transformation: ΔH = ΔH1+ΔH2+ΔH3.