Where are the chloroplasts located within the elodea cell.
Answers
Chloroplasts are located in the cytoplasm of plant cells. Specifically, chloroplasts are found within the mesophyll cells of the leaf, which are located near the surface of the leaf where light can be absorbed.
Elodea is an aquatic plant that is often used in science experiments to study photosynthesis. It has transparent cells that make it easy to observe the chloroplasts and their movements during photosynthesis. In the elodea cell, the chloroplasts are located in the cytoplasm, and they are responsible for carrying out photosynthesis.
During this process, they absorb light energy and convert it into chemical energy in the form of glucose, which is used by the cell as fuel.
To learn more about Chloroplasts visit;
https://brainly.com/question/11136550
#SPJ11
Related Questions
What is the name of the compound Si₉Br₃?
Answers
The identity of the element shown in the model above is determined by the number of -

Answers
Answer:
The number of protons or electrons in its atomic structure
Explanation:
Elements are substances which constitute the building block of matter. The smallest particles of elements that can ever exist are known as atoms. Atoms of different elements are different in their structure properties. This difference is due to to the number of sub-atomic particles present in the atom of each element.
The three sub-atomicparticles are protons, neutrons and electrons. Protons and neutrons are present in the nucleus of an atom, while the electrons revolve around the nucleus in orbits. The identity of each element is determined by the number of protons present in the atomic nucleus of that element. This is known as the atomic number of that element. For neutral atoms, the proton number is equal to electron number.
For the given element, the electron and proton number are respectively 4, therefore, the atomic number of the element is 4. The element with atomic number 4 is Beryllium.
TRUE/FALSE. the phosphate attached to the 5' carbon of a given nucleotide links to the 3' -oh of the adjacent nucleotide.
Answers
The phrase, "the phosphate attached to the 5' carbon of a given nucleotide links to the 3' -OH of the adjacent nucleotide." is true.
In the context of nucleic acids like DNA and RNA, nucleotides are the building blocks that consist of a sugar molecule, a phosphate group, and a nitrogenous base. The nucleotide has its phosphate group linked to the carbon atom in the sugar molecule at the 5' position.
The nucleotides are linked together to form a polynucleotide chain through phosphodiester bonds. These bonds are formed between the 3' -OH group of one nucleotide and the phosphate group of the adjacent nucleotide, which is attached to the 5' carbon.
The formation of the phosphodiester bond involves a condensation reaction, where a water molecule is released as a result of the bond formation. This linkage creates a backbone of sugar-phosphate units with the nitrogenous bases projecting outwards.
In summary, it is true that the phosphate attached to the 5' carbon of a given nucleotide links to the 3' -OH of the adjacent nucleotide, creating the characteristic sugar-phosphate backbone found in nucleic acids.
Learn more about nucleotide at: https://brainly.com/question/1569358
#SPJ11
Would the compound H2Se be expected to form intermolecular hydrogen bonds in the liquid state? Yes • No
Answers
No, the compound H2Se (hydrogen selenide) would not be expected to form intermolecular hydrogen bonds in the liquid state. To understand why, let's first define the terms involved:
1. H2Se: Hydrogen selenide is a covalent compound composed of two hydrogen atoms bonded to one selenium atom.
2. Intermolecular: Referring to interactions between separate molecules rather than within the same molecule.
3. Hydrogen bond: A type of attractive force that occurs between a hydrogen atom covalently bonded to a highly electronegative element (such as nitrogen, oxygen, or fluorine) and a lone pair of electrons on another electronegative element.
In the case of H2Se, the hydrogen atoms are bonded to a selenium atom. Although selenium is an electronegative element, it is not as highly electronegative as nitrogen, oxygen, or fluorine. The electronegativity difference between hydrogen and selenium is not significant enough to result in the formation of a strong partial positive charge on the hydrogen atoms, which is necessary for the formation of intermolecular hydrogen bonds.
As a result, in the liquid state, H2Se molecules interact with one another through weaker forces such as dipole-dipole interactions and London dispersion forces, but they do not form hydrogen bonds. These weaker intermolecular forces contribute to H2Se having a lower boiling point than compounds that do exhibit hydrogen bonding.
for more information on H2Se see:
https://brainly.com/question/31020856
#SPJ11
what are some importance of density?
Answers
Answer:
allows us to determine what substances will float and what substances will sink when placed in a liquid
Explanation:
Generally, substances float so long as their density is less than the density of the liquid they are placed in.
if the system has more than one solution, then so does the homogeneous system group of answer choices true false
Answers
The Trivial Solution is what we refer to as. A homogeneous system can never be inconsistent since it always has a solution (the simple solution). As a result, a homogeneous system of equations always has either a singular solution.
True or false: Is it possible for a system to have multiple solutions?A system of linear equations typically has a single solution, although it is occasionally possible for there to be no solution (parallel lines) or infinitely many solutions (same line).
What characteristics distinguish a homogeneous system?When all of the constant terms in a system of linear equations are equal to zero, the system is said to be homogeneous. A homogeneous system always has at least one solution, which is the zero vector. A homogeneous system that has undergone a row operation remains homogenous after the operation.
To know more about homogeneous system visit :-
https://brainly.com/question/13110297
#SPJ4
the nickel anode in an electrolytic cell decreases in mass by 1.20 g in 35.5 min. the oxidation half-reaction converts nickel atoms to nickel(ii) ions. what is the constant current
Answers
The constant current is 0.0406 A for the nickel anode in an electrolytic cell decreases in mass by 1.20 g in 35.5 min. the oxidation half-reaction converts nickel atoms to nickel(ii) ions.
What is the constant current?In an electrolytic cell, the oxidation half-reaction converts nickel atoms to nickel (II) ions, and the nickel anode in an electrolytic cell decreases in mass by 1.20 g in 35.5 min.
To determine the constant current, we can use Faraday's laws. Faraday's laws were established by Michael Faraday, a British scientist, in the early 19th century. His laws explain how much mass will be lost or gained at an electrode during electrolysis and how much electrical energy is required. Faraday's first law states that the mass of a substance deposited during electrolysis is proportional to the number of electrons that pass through the electrolyte.
The following formula can be used to calculate the constant current:
I = (nF / t) × (m / M)
where, I = Constant Current (in amperes), n = number of moles of electrons transferred, F = Faraday constant (96500 C/mol), t = Time taken, m = mass of substance (in grams), M = Molar mass of the substance (in grams/mol)
The Faraday constant is the amount of charge that must pass through an electrode to deposit or liberate 1 mole of any substance. For nickel, the molar mass is 58.69 g/mol, and the oxidation state is +2, which means that two electrons are lost per nickel atom. Thus, n = 2.
To calculate the current, we must first find the number of moles of nickel atoms lost during electrolysis. The formula for the number of moles is:
n = m / M
n = 1.20 g / 58.69 g/mol
n = 0.0204 mol.
Now we can use the formula above to calculate the current:
I = (nF / t) × (m / M)
I = (2 × 96500 C/mol / 2130 seconds) × (1.20 g / 58.69 g/mol)
I = 0.0406 A
I = 40.6 mA or 0.0406 A.
Therefore, the constant current is 40.6 mA or 0.0406 A.
Read more about current here:
https://brainly.com/question/12815553
#SPJ11
a regular solid box has a length of 5cm a width of 2cm and a height of 3cm what is the volume of the box
Answers
Volume of the regular solid = l × w × h
= (5 cm × 2 cm × 3 cm)
= 30 cm³
What would you call the following chemical reaction, Zn(s) + H2O(g) – Zno (s) + H2(g)
O double replacement/displacement
single replacement/displacement
O combustion
O decomposition/analysis
O synthesis/combination

Answers
I'd say it's single replacement/displacement
Please help
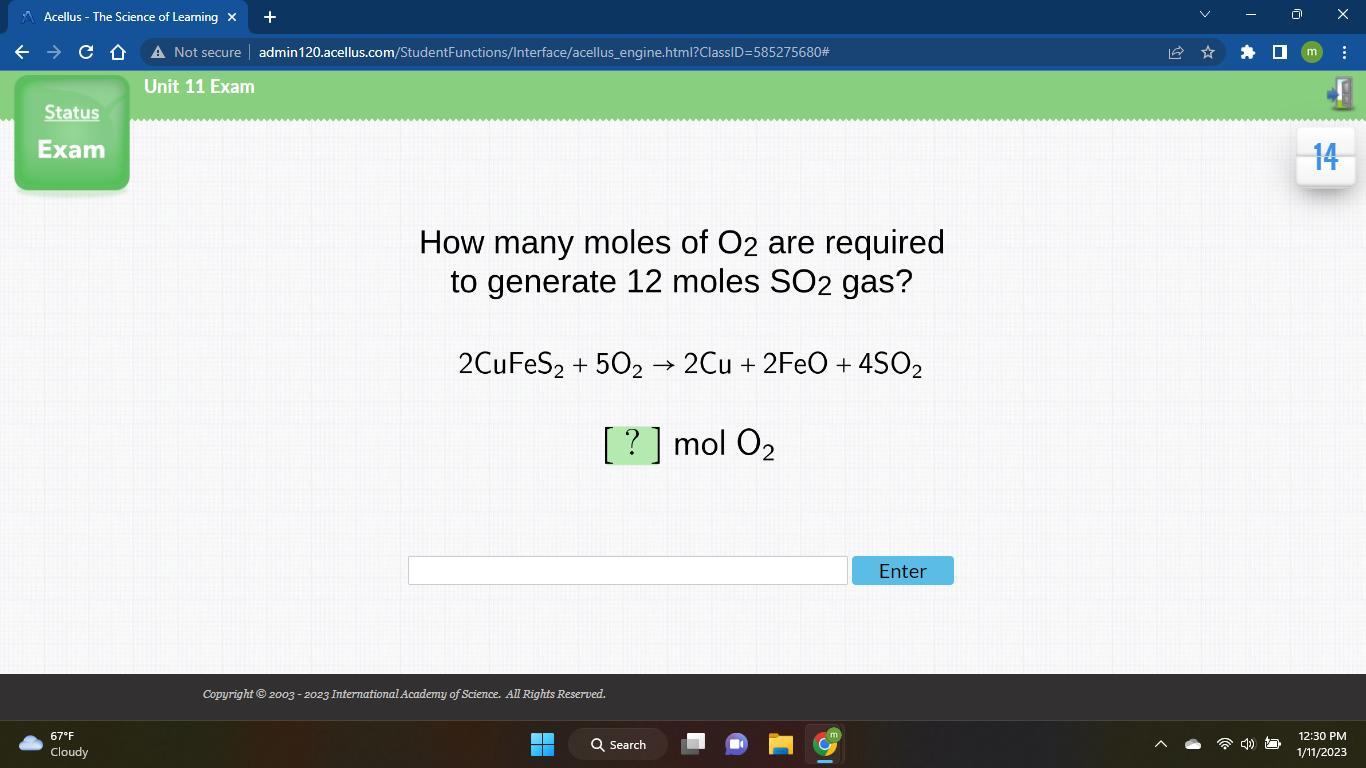
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
The mixing of which pair of reactants will result in a precipitation reaction?
NH4Cl(aq) + NH4I(aq)
KClO4(aq) + (NH4)2S(aq)
Na2SO4(aq) + Cu(NO3)2(aq)
Ba(NO3)2(aq) + K2CO3(aq)
Answers
Answer:
The last option will form the precipitate reaction of insoluble compound
the manipulated variable in this experiment is the
a) type of antacid
b) amount of antacid used
c) time it takes for the reaction to occur
d) temperature at which the reaction occurs
Answers
The independent variable, also known as manipulated, is the one that changes and causes an effect on the depenedent variable. The manipulated variable is temperature. Option D.
What is the independent (manipulated) variable?Independent variables are those modified or changed by the researcher to study how this change affects another variable (the dependent one) and hence the results.
It also receives the name of the manipulated variable because the researcher alters its value or state to analyze its effect. These variables are voluntarily manipulated by the researcher.
In the exposed example,
Goal: To determine the effect of temperature on reaction rates.
Independent (manipulated) variable: Temperature
Dependent variable: time it takes the tablet to dissolve.
Temperature is what affects the time in which the tablet dissolves. The researcher changes temperature levels to analyze how much the time changes.
You can learn more about independent variables at
https://brainly.com/question/1479694
#SPJ1
Complete question,
A group of students conducts an experiment to determine the effect of temperature on reaction rates. They perform three separate trials in this experiment.
In the first trial, they drop an antacid tablet into a beaker of water at a temperature of 40 °C and record how long it takes the tablet to completely dissolve.
In the second and third trials, they use the same type and amount of antacid, but they change the temperature of the water to 25 °C for the second trial and 5 °C for the third trial.
The manipulated variable in this experiment is the
A. type of antacid used
B. amount of antacid used
C. time it takes for the reaction to occur
D. temperature at which the reaction occurs
mass of 2 into 10 to power 21 number of atoms of an element is 0.4 gram what is the mass of 0.5 mole of the elements
Answers
The mass of 0.5 mole of the element is approximately 6.025 grams.
To calculate the mass of 0.5 mole of the element, we need to know the molar mass of the element.
Given that the mass of 2 x 10^21 atoms of the element is 0.4 grams, we can use this information to find the molar mass.
The number of atoms in 1 mole of any substance is given by Avogadro's number, which is approximately 6.022 x 10^23 atoms/mol.
First, we calculate the molar mass of the element using the given information:
Molar mass = Mass of 2 x 10^21 atoms / Number of moles of 2 x 10^21 atoms
Molar mass = 0.4 g / (2 x 10^21 atoms / (6.022 x 10^23 atoms/mol))
Molar mass ≈ 0.4 g / (3.32 x 10^-2 mol)
Molar mass ≈ 12.05 g/mol
Now that we know the molar mass of the element is approximately 12.05 g/mol, we can calculate the mass of 0.5 mole of the element:
Mass = Molar mass x Number of moles
Mass = 12.05 g/mol x 0.5 mol
Mass = 6.025 grams
for more such questions on element
https://brainly.com/question/28376204
#SPJ8
Discussion Topic
Both Josef Loschmidt and Amedeo Avogadro contributed to our understanding of basic
molecular numbers, sizes, and reaction ratios. Neither scientist discovered Avogadro's
number in the form we use it today (6.02 x 10^23). Still, there's controversy over the
name of this number. Research the contributions of these two scientists and how
Avogadro's number got its name. Note the name you think this number should becalled, provide key details about each scientist's contributions to this concept, and give
a solid rationale for your case in naming the number.

Answers
Josef Loschmidt and Amedeo Avogadro were both scientists who made significant contributions to the understanding of basic molecular numbers, sizes, and reaction ratios.
What is the rational behind their contributions?In 1811, Amedeo Avogadro proposed that equal volumes of gases at the same temperature and pressure contain the same number of molecules. This became known as Avogadro's law, which laid the foundation for the concept of the mole.
Josef Loschmidt, on the other hand, made important contributions to determining the size of molecules. In 1865, Loschmidt used kinetic theory to calculate the number of molecules in one cubic centimeter of gas at standard temperature and pressure (STP). He estimated the number to be about 2.7 x 10¹⁹, which is close to the modern value of Avogadro's number.
The term "Avogadro's number" was not coined until the early 1900s, long after Avogadro's death. The name was proposed by French physicist Jean Baptiste Perrin in honor of Avogadro's contributions to the concept of the mole.
In my opinion, the name "Avogadro's number" is appropriate because Avogadro's law was the first concept to establish a relationship between the volume of a gas and the number of molecules it contains. Moreover, Avogadro's law played a crucial role in the development of the mole concept, which is essential in chemical calculations. While Loschmidt's contributions were also significant, he did not propose a fundamental law like Avogadro did. Therefore, I believe that naming the number after Avogadro is appropriate to recognize his contributions to this fundamental concept in chemistry.
Learn more on Avogadro here: https://brainly.com/question/1513182
#SPJ1
QUESTION WITH ANSWER...
Which system is used to measure physical properties? Most scientists use the (BLANK) system, also known as the metric system, to measure physical properties of elements.
THE ANSWER IS: Standard International system
Answers
Standard International system is a system is used to measure physical properties.
Which system is used to measure physical properties?
The International System of Units (SI), is commonly known as the metric system. This system is used as the international standard for measurement. Most scientists use the International System of Units which is also known as the metric system in order to measure physical properties of elements. The metric system is a system of measurement that uses meter as base units of length (distance), liter for volume, and , and gram for weight or mass. We use units that are derived from the metric units in order to measure smaller or larger quantities.
So we can conclude that Standard International system is a system is used to measure physical properties.
Learn more about physical properties here: https://brainly.com/question/12330204
#SPJ1
HELP PLEASE 1pt
Identify the object that has the greatest gravitational pull.
A.
a tennis ball
B.
the moon
C.
the sun
D.
Earth

Answers
The object with the greatest gravitational pull is the Earth (option D).
What is gravitational pull?Gravitational force is a very long-range, but relatively weak fundamental force of attraction that acts between all particles that have mass. It is believed to be mediated by gravitons.
Newton’s Law of Universal Gravitation states that every particle attracts every other particle in the universe with force directly proportional to the product of the masses and inversely proportional to the square of the distance between them.
The force of gravitational attraction is directly dependent upon the masses of both objects and inversely proportional to the square of the distance that separates their centers.
So as the mass of either object increases, the force of gravitational attraction between them also increases. If the mass of one of the objects is doubled, then the force of gravity between them is doubled. If the mass of one of the objects is tripled, then the force of gravity between them is tripled.
According to this question, Earth has the highest mass among the listed objects, hence, in accordance to the law of universal gravitation, Earth will possess the greatest gravitational pull.
Learn more about gravitational pull at: https://brainly.com/question/13467280
#SPJ1
Which explains the change in ionization energy that occurs between removing the first and second electrons from an atom?
Answers
Answer:
"The strength of ionization decreases as the proportion including its protons to the excited electrons" is the right solution.
Explanation:
The ionizing procedure, the creation of ions through manipulating individual atoms or revolutionaries, or through decreasing or increasing charged particles in something other than gas by intense electrical fields.It should be the total removal of someone with an electron through an atomic nucleus rather than from molecules. The corresponding molecule has denominated an ion.This question seems incomplete. I believe the answer choices are as followed:
O The ionization energy decreases because the ratio of the protons to electrons increases.
O The ionization energy increases because the ratio of the protons to electrons increases.
O The ionization energy decreases because the ratio of the protons to electrons decreases.
O The ionization energy increases because the ratio of the protons to electrons decreases.
The answer to this is The ionization energy increases because the ratio of the protons to electrons increases. (2nd option).
Find how much mass is in 2.1 moles H2O
Answers
2.1 moles of \(H_2O\) will have a mass of 37.8 grams.
Moles and masses of substancesThe number of moles a substance contains and the mass of the substance are related by the following equation:
Mole = mass/molar mass
In other words, the mole of a substance is the ratio of the mass of the substance and its molar mass.
Rearranging the equation:
Mass = mole x molar mass.
In this case, we want to find the mass of 2.1 moles of water. The molar mass of water can be calculated as follows:
\(H_2O\) = (1 x 2) + 16
= 18 g/mol
Mass of 2.1 moles of water = 2.1 x 18
= 37.8 grams
In other words, 2.1 moles of water will have a mass of 37.8 grams.
More on moles of substances can be found here: https://brainly.com/question/26416088
#SPJ1
The pH scale was designed to make it convenient to express hydrogen ion concentrations that are small in aqueous solutions. The definiton of pH is in terms of base 10 logarithms.

Answers
Answer:
a. pH = 2.22.
b. [H+] = 2.588 x 10⁻⁴ mol/L.
Explanation:
Acids and Bases => Calculating pH of Acids and Bases.
As we saw before, the formulas to find the pH based on the hydrogen ion concentration [H+], and to find the hydrogen ion concentration [H+] based on the pH are the following, respectively:
\(\begin{gathered} pH=-log\lbrack H^+], \\ \\ [H^+]=10^{-pH}. \end{gathered}\)So let's see each case:
a. To find the pH of an H+ concentration of 6.02 x 10⁻³ mol/L we use the pH formula:
\(\begin{gathered} pH=-log\lbrack6.02\cdot10^{-3}], \\ \\ pH=2.220\approx2.22. \end{gathered}\)The answer would be that the pH is 2.22.
b. To find the H+ concentration of a pH of 3.587, we use the [H+] formula:
\(\begin{gathered} \lbrack H{}^+]=10^{-3.587}, \\ \\ [H^+]=2.5882\cdot10^{-4}\text{ mol/L}\approx2.588\cdot10^{-4}\text{ mol/L.} \end{gathered}\)The answer would be that the hydrogen ion concentration [H+] = 2.588 x 10⁻⁴ mol/L.
PLEASE ANSER QUICK ITS URGENT 40 POINTS
Scuba tanks are often pressurized to about 204 atm before the start of the dive.
What is the pressure of 204 atm in kPa?
[ ? ] kPa
Round your answer to three significant figures.
Pressure (kPa)

Answers
Answer:
1 atm = 101.325 kPa
Therefore, 204 atm = 204 * 101.325 = 20670.3 kPa
Rounded to three significant figures, the pressure of 204 atm is 2.07 x 10^4 kPa.
an exothermic chemical reaction between a solid and a liquid results in gaseous products. spontaneous?
Answers
An exothermic chemical reaction between a solid and a liquid results in gaseous products. It is a spontaneous reaction.
What is an exothermic reaction?When a chemical reaction takes place with the release of heat, it is known as an exothermic chemical reaction. An exothermic chemical reaction is a chemical reaction that releases energy in the form of heat, light, or sound during the process. The burning of paper is an example of an exothermic chemical reaction. When paper burns, heat and light are produced, which we can feel or observe.
The reaction is spontaneous if the Gibbs free energy, delta G is negative. A reaction will be spontaneous if its delta G is negative. The reaction will proceed from left to right if delta G is negative, and it will proceed from right to left if delta G is positive. A reaction will be at equilibrium if delta G is zero.The reaction mentioned in the question is an exothermic chemical reaction because it results in the release of heat. As a result, the reaction is spontaneous. The production of gaseous products indicates that a gas is formed during the reaction. Therefore, this reaction is spontaneous.
Learn more about Exothermic reaction here:
https://brainly.com/question/10373907
#SPJ11
Rank these ions according to ionic radius. Largest radius Ca^2+ P^3-
S^2- CI^- K^+ Smallest radius Rank these ions according to ionic radius. Is this correct?
Answers
The correct order for Ionic radii in decreasing order in the following order: P3- > S2- > Cl- > K+ > Ca2+.
Here, we see that all species have the same number of electrons—each has 18 electrons—after gaining (in the case of anions) or losing (in the case of cations) the appropriate number of charges over them.
So, in this case, the electrostatic force between the nucleus and the shell-bound electrons is used to determine the ionic radius.
For example, a nucleus with more protons will have a stronger electrostatic force and a smaller ionic radius.
Ionic radii are in decreasing order in the following order: P3- > S2- > Cl- > K+ > Ca2+
To learn more about ionic radii click here:
https://brainly.com/question/8137711
#SPJ4
What is a sensitive device used to detect magnetic fields on the seafloor?
A. seismometer
B. geologist's compass
C. Glomar
D. magnetometer
Answers
Answer:
D. Magnetometer
Explanation:
A magnetometer is a device that measures magnetic field or magnetic dipole moment. Some magnetometers measure the direction, strength, or relative change of a magnetic field at a particular location.
Study the reactions for the formation of compounds from their elements. I. C(s) + O2(g) → CO2(g) ΔHf = −394 kJ II. H2(g) + 12O2(g) → H2O(l) ΔHf = −242 kJ III. 2C(s) + 3H2(g) → C2H6(g) ΔH =−84 kJ The combustion of C2H6 is shown by the following equation: C2H6(g) + 72O2(g) → 2CO2(g) + 3H2O(l) Which option correctly gives the enthalpy of combustion of 0.2 moles of C2H6(g)? −1,430 kJ 286 kJ −286 kJ 1,430 kJ Exam 3 Click on the numbers to jump from one question to another. 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 16 17 18 19 20 21 22 23 24 25
Answers
Answer:
The correct option is -286 kJ
Explanation:
The given parameters are
C(s) + O₂(g) → CO₂(g) ΔHf = -394 kJ
H₂(g) + 12O₂(g)→H₂O ΔHf = -242 kJ
2C(s) + 3H₂(g)→C₂H₆(g) ΔH = -84 kJ
Te given equation is C₂H₆(g) + 7/2O₂(g) →2CO₂(g) + 3H₂O(l)
The heat of formation or enthalpy of combustion = Heat of formation of the products - Heat of formation of the reactants
The enthalpy of combustion of the reaction = 2*(-394) + 3*(-242)- ((-84)+7/2*0)) = -1,430 kJ
Given that the reaction consists of one mole of C₂H₆(g), we have;
The enthalpy of combustion of one mole of C₂H₆(g) = -1,430 kJ
Therefore, the enthalpy of combustion of 0.2 mole of C₂H₆(g) = -1,430 kJ × 0.2 = -286 kJ
The correct option = -286 kJ.
Answer:
Positive 1,430
Explanation:
When the first American astronauts were planning to walk on the Moon, they knew that the gravity on the Moon was less than the gravity on Earth. With this information, what did the astronauts expect to be MOST different on the Moon? Select one: a. their mass b. their height c. their weight d. their volume
Answers
Answer:
Their weight.
Explanation:
Weight is the measurement of gravity pushing you down, so with less gravity there would be less weight.
The astronauts expect their weight to be different on the Moon
Definition of weightWeight is simply defined as the gravitational pull of the earth on an object. It is measured in Newton.
Description of weightThe weight of an object varies with location because of gravity.
The weight of an object is related to it's mass according to the following equation:
Weight = mass × Acceleration due to gravity
Since the gravity of the Moon is lesser than that of the Eearth, the astronauts certainly knew that their weight will be different on the Moon.
Learn more about weight:
https://brainly.com/question/1839641
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m
2
. How much thrust both engines are generating?
Answers
A twin-turbojet airplane is cruising with a speed of Mach 1.5 at an altitude where atmospheric pressure is 32989.5 Pa, temperature is 232.778 K. Each engine is consuming 200 kg of air per second. The engine exit flow has a pressure of 32,000 Pa with a velocity of 850 m/sec. The exit area of the engine nozzle is 1.4 m². The thrust generating in both engines is 342,678.6 Newtons.
To calculate the thrust generated by both engines, we can use the momentum equation for a nozzle:
Thrust = mass flow rate * exit velocity + (exit pressure - ambient pressure) * exit area
Given:
Speed of the airplane (V) = Mach 1.5
Atmospheric pressure (\(P_a\)) = 32989.5 Pa
Ambient temperature (\(T_a\)) = 232.778 K
Mass flow rate of each engine (m) = 200 kg/s
Exit pressure of the engine (\(P_e\)) = 32000 Pa
Exit velocity of the engine (\(V_e\)) = 850 m/s
Exit area of the engine nozzle (\(A_e\)) = 1.4 m²
First, we need to calculate the ambient density using the ideal gas law:
PV = nRT
Since the speed of the airplane is given in terms of Mach number, we can calculate the speed of sound (a) using the following formula:
a = √(gamma * R * \(T_a\))
Where gamma is the specific heat ratio of air (approximately 1.4) and R is the specific gas constant for air (approximately 287 J/(kg K)).
Next, we can calculate the ambient density (ρ) using the equation:
ρ = \(P_a / (R * T_a)\)
Now, we can calculate the thrust generated by each engine using the momentum equation:
Thrust = m* \(V_e + (P_e - P_a) * A_e\)
Finally, we can calculate the total thrust generated by both engines by multiplying the thrust of a single engine by 2.
Calculate the speed of sound:
a = √(1.4 * 287 * 232.778)
a = 438.95 m/s
Calculate the ambient density:
ρ = 32989.5 / (287 * 232.778)
ρ = 1.383 kg/m³
Calculate the thrust of a single engine:
\(Thrust_s\) = 200 * 850 + (32000 - 32989.5) * 1.4
\(Thrust_s\) = 170000 + 1339.3
\(Thrust_s\) 171339.3 N
Calculate the total thrust of both engines:
\(Thrust_t\) = 2 * \(Thrust_s\)
\(Thrust_t\) = 2 * 171339.3
\(Thrust_t\) = 342678.6 N
Therefore, both engines are generating approximately 342,678.6 Newtons of thrust.
To know more about thrust here
https://brainly.com/question/26712174
#SPJ4
Given the system at equilibrium:
H3PO4 + 3 H2O <-----> 3 H3O+ + PO4^3-
If Na3PO4(s) is added, there will be a decrease in the
concentration of
A) Na+
B) PO4^3–
C) H3O+
D) H2O
Answers
Answer:
Adding Na3PO4(s) will introduce more PO4^3- ions into the solution, which will react with H3O+ ions to form more H3PO4 and H2O through the reverse reaction. This will shift the equilibrium to the left, decreasing the concentration of H3O+ ions and increasing the concentration of H3PO4 and H2O. Therefore, the concentration of H3O+ ions will decrease, and the correct answer is (C) H3O+.
KOH + CO3(PO4)2 →
4 8
K3PO4 +
—
2
CO(OH)2
48
Answers
6KOH + CO₃(PO₄)₂ → 2K₃PO₄ + 3CO(OH)₂
It is a double displacement reaction.
Double displacement reaction- In aqueous solutions, where ions precipitate and exchange occurs, double displacement processes are most common. For instance, a white precipitate of barium sulphate quickly forms when a solution of barium chloride and sodium sulphate are combined. These are ionic-based reactions.
In double displacement reactions, there is simply an ion exchange between the two reactants and no oxidation or reduction of any of the reactants. Double displacement processes are therefore not redox processes.
The positive ions trade partners with the negative ions during double displacement processes. Ionic chemicals that are dissolved in water undergo several double displacement reactions with one another.
Thus it is a double displacement reaction.
To learn more about double displacement reaction refer- https://brainly.com/question/23918356
#SPJ9
Which of the following trends can be observed when moving down a column in the Periodic Table?
A.) The atomic mass decreases.
B.) The number of valence electrons remains the same.
C.) The number of orbitals decreases.
D.) The possible number of bonds formed decreases.
Answers
The trend that can be observed when moving down a column in the periodic table is: B.) The number of valence electrons remains the same.
Diagram of a periodical table showing the arrangement of elements according to their properties is shown in the attachment below.
Recall the following that can be noticed as you go down through the column in the periodic table:
As we go down the column, for example, the first column, atomic mass increase. Atomic mass of the element down below is more than the atomic mass of the elements above it in the same column.Valence electrons for all the elements within a column is the same number found outside their shell which defines the type of chemical bond they can form with another atom.The number of orbitals increases as we progress down the column in a periodic table, since number of atomic number also increases down the column.Number of bonds an element can formed depends on the number of valence electrons it has. Since all elements within a group (column) are the same, possible number of bonds that elements within a column can form will be the same.Therefore, we can conclude that the trend that can be observed when moving down a column in the periodic table is: B.) The number of valence electrons remains the same.
Learn more here:
https://brainly.com/question/9621716

Answer:
B ' The number of valence electrons remains the same.'
Explanation:
4. How does the
productivity of
photosynthesis affect the
productivity of cellular
respiration?
Answers
Answer: it’s the chemicals between the biological
Explanation:
The productivity of photosynthesis affect the productivity of cellular respiration by providing energy.
What is photosynthesis?Photosynthesis is a process where plants prepare their food in the presence of sunlight.
Photosynthesis process chemically illustrated as :
6CO₂ + 6H₂O → C₆H₁₂O₆ + 6O₂
From the above it is clear that carbohydrate is produced in the photosynthesis process and this carbohydrate provide energy to the plant for the completion of another chemical process. So, for the cellular respiration plants use this energy.
Hence, the productivity of photosynthesis directly affect the productivity of cellular respiration.
To learn more about photosynthesis, visit the below link:
https://brainly.com/question/25869814