Which ion in each pair has greater charge density? Explain.
(a) Na⁺ or Cs⁺
Answers
Na⁺ has a greater charge density Cs⁺.
The accumulation of electric charge in a specific field is measured by the charge density. It gauges electric charge according to the following dimensions. The distribution of electric charge determines the charge density, which can be either positive or negative. The measure of electric charge per unit area of a surface, or per unit volume of a body or field, is called charge density.
How much electric charge has accumulated in a particular field is indicated by its charge density. The main thing it determines is the charge density per unit of length, surface area, and volume. It calculates the amount of electricity used per square foot of space. The dimensions of this area could be one, two, or three. The position, which may be negative, will determine the charge density.
To know more about charge density refer to: https://brainly.com/question/12968377
#SPJ4
Related Questions
In what way was the reaction of the splint and CO2 different from the reaction of the H2 to the flaming splint
Answers
Explain to the kids that since there is essentially no —which is required for fire—if the bag contains only pure carbon dioxide, the splint would burn out right away.
What occurs when a burning splint is placed in hydrogen?H2 - Hydrogen Pure hydrogen gas will burst into flames when a burning splint is added to it, making a popping sound. Oxygen (O2) A smouldering splint will rekindle when exposed to a sample of pure oxygen gas.
The flame goes out as a result of carbon dioxide replacing the oxygen it requires to burn (the effect). A popping sound is produced when a flame is near hydrogen because of how the gas burns.
learn more about flaming splint
https://brainly.com/question/30124568
#SPJ1
A student was given a sample of food and asked to determine the types of nutrients present in the sample. The student placed half of the sample in a test tube with Benedict’s solution and heated it. The solution turned brick red. When an iodine solution was added to the remaining half of the sample, it turned blue black. The student can correctly conclude that the food sample contained
Answers
The food sample contained starch and reducing sugar (carbohydrates).
The Benedict's test is used to test for the presence of reducing sugars, such as glucose, in a sample. When the Benedict's solution is added to a sample containing reducing sugars and heated, the solution will turn brick red.
The iodine test is used to test for the presence of starch in a sample. When iodine solution is added to a sample containing starch, it will turn blue-black.
So, in this case, the student can conclude that the food sample contained both starch and reducing sugars, as both tests produced positive results.
Learn more about Benedict's test and Iodine test here: https://brainly.com/question/25800056
#SPJ4
how many carbon atoms are in 10.0mg of aspirin C9H8O4 molar mass
180 g mol-1
Answers
There are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
The molar mass of aspirin (C9H8O4) is 180 g/mol. Calculate the number of carbon atoms in 10.0 mg of aspirin. The molar mass of C9H8O4 = 9 x atomic mass of C + 8 x atomic mass of H + 4 x atomic mass of O= 9 x 12.011 + 8 x 1.008 + 4 x 15.999= 180.16 g/mol.
Hence, 1 mole of aspirin weighs 180.16 g and contains 9 moles of carbon atoms (1 mole of C9H8O4 contains 9 carbon atoms). Number of moles of aspirin in 10.0 mg = 10.0 mg/180.16 g/mol= 0.0000555 mol. Number of carbon atoms in 10.0 mg of aspirin= 9 x 0.0000555= 0.0004995.
Therefore, there are approximately 0.0004995 carbon atoms in 10.0 mg of aspirin.
Learn more about the "carbon atoms" :
https://brainly.com/question/17154602
#SPJ11
what gas (name and formula) is produced in the chemical reaction of iron metal and hydrochloric acid
Answers
Which statement describes chemical properties?
A. Characteristics that can change without changing the substance
B. Characteristics that can change when the state of the substance
changes
C. Characteristics that describe the ability of a substance to form a
new substance
D. Characteristics that describe what chemical a substance is made
up of
QQ
Answers
Answer:
D
Explanation:
A substance is made up of chemicals
Help! Help ASAP!
Using bonding principles, describe why AlCl3 forms an ionic bond
Answers
Simple
Look at Electronic configuration of Al
[Ne]3s²3p¹It has valency as 3
So it can make 3 bonds
Chlorine has well known valency 1So
Aluminium does bonding with 3 chlorine atoms
There is another reason
That's electronegativity
Chlorine is most electronegative elementAluminium has low electronegativityAs difference in electronegativity is higher they forms ionic bond
describe where
the energy is coming from and how it is affecting change or putting an object into motion
help me and fast

Answers
The energy is coming from the battery that is in the torchlight.
In the case of an object in motion, energy is coming from the kinetic energy of the object. Kinetic energy is the energy an object possesses due to its motion. This energy is transferred to the object by an external force, such as a push or a pull, which causes the object to start moving or to change its motion.
What is the energy about?Energy is the ability to do work and can take many forms, such as thermal, kinetic, potential, and chemical energy. Energy can be transformed from one form to another and can also be transferred from one object to another.
Therefore, In the case of an object being put into motion, energy is coming from the potential energy of the object. Potential energy is the energy an object possesses due to its position or configuration. This energy is transferred to the object by an external force, such as gravity, which causes the object to start moving or to change its motion.
Learn more about energy from
https://brainly.com/question/2003548
#SPJ1
Matter is classified as a
A.homogeneous or heterogeneous mixture
B.substance or a mixture of substances
C.substance, only
D.mixture of compounds
Answers
Matter is classified as a substance or a mixture of substances.
Pure substances and mixtures are the two subgroups of matter. Elements and compounds are formed through the further division of pure substances.
Physically coupled structures known as mixtures can be disassembled into their component parts. One particular sort of atom or molecule makes up a chemical compound.
At what speed does every type of electromagnetic radiation travel?
Answers
Answer:
All waves of the electromagnetic spectrum have a speed of 3 × 10⁸ ms⁻¹
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
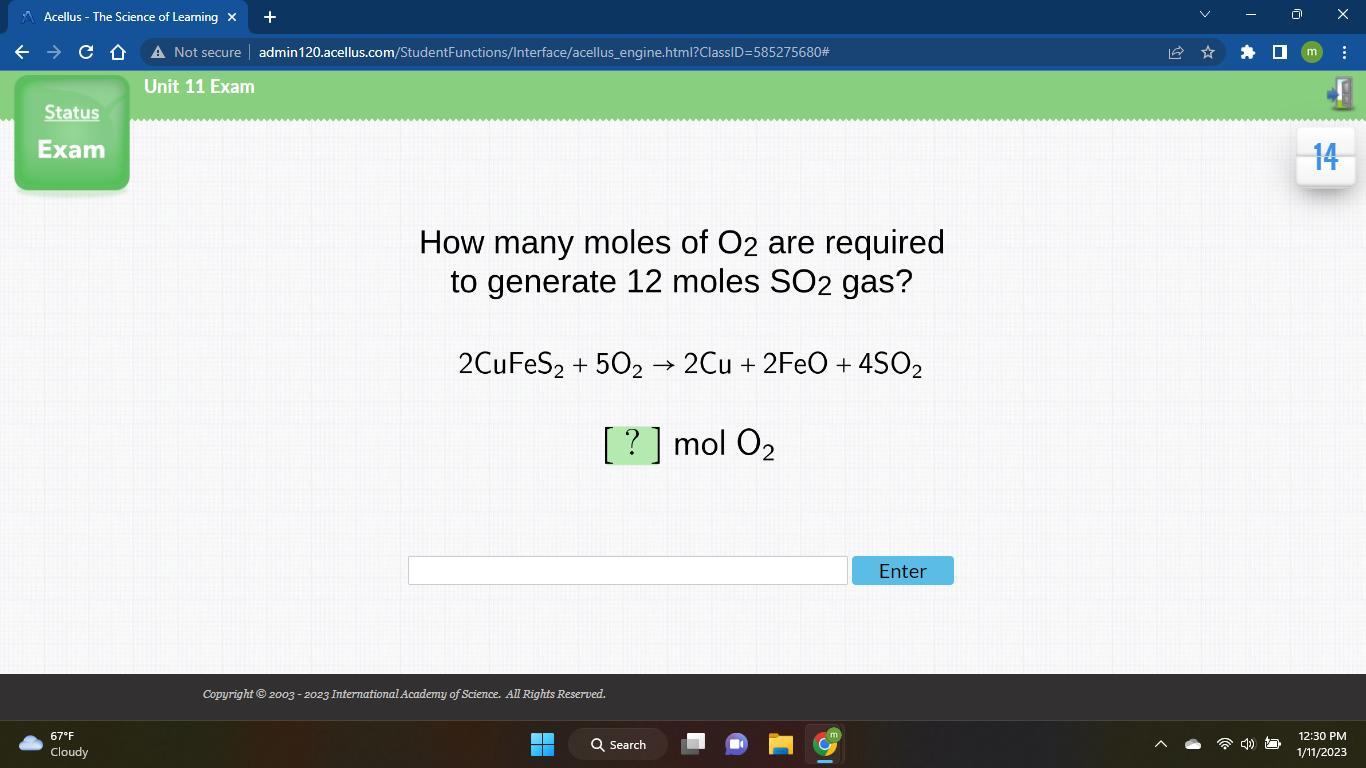
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
Which statement would most likely be found in an advertisement from a cell phone provider?
Cell phone use may result in damage to brain tissue.
The number of cell phone users increases every year.
Cell phones emit potentially harmful radio waves.
Answers
the second option would most likely be found in a cell phone advertisement!
Answer: the answer is b
Explanation: cuz i said so
a student needs to prepare 100. ml of a 0.50 m naoh solution from a 2.0 m naoh stock solution. what volume of the stock solution should she transfer to a 100. ml volumetric flask ? show the calculation. g
Answers
The volume of the stock solution she should transfer to a 100 ml volumetric flask is 25 ml.
Dilution is defined as the process in which the concentration of a sample is decreased by adding more solvent. The dilution formula is given below.
C₁V₁ = C₂V₂
where C₁ = initial concentration of sample = 2.0 m
V₁ = initial volume of sample
C₂ = final concentration after dilution = 0.50 m
V₂ = total final volume after dilution = 100 ml
Plug in the values to the formula and solve for the volume of the stock solution needed in the dilution.
C₁V₁ = C₂V₂
V₁ = C₂V₂/C₁
V₁ = (0.50 m)(100 ml)/(2.0 m)
V₁ = 25 ml
Learn more about dilution here: brainly.com/question/1615979
#SPJ4
How does the human body build the complex
molecules it needs?
Answers
Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break molecules down and generally release energy. Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case
Answer:
Dehydration synthesis reactions build molecules up and generally require energy, while hydrolysis reactions break molecules down and generally release energy. Carbohydrates, proteins, and nucleic acids are built up and broken down via these types of reactions, although the monomers involved are different in each case.
Explanation:
Gases that are abundantly emitted by volcanoes include ________. Group of answer choices helium carbon dioxide water vapor sulfur dioxide or hydrogen sulfide
Answers
Answer:
Water vapour (more than 60%)
Which element, oxygen or fluorine, can steal an electron more easily? Explain why.
Answers
Answer:
Answer Choice, Oxygen
Explanation:
Oxygen, because it is more up and to the right than any of the other elements listed, so it has more electronegativity and will try to steal more electrons to fill up its valence orbitals.
Al agregar 150g de una sustancia X en un recipiente que sostiene que contiene agua hasta 50, el nivel del agua aumenta hasta 120ml. Calcula la densidad de la sustancia X
Answers
Answer:
2,14 g / ml
Explanation:
Sabemos que el volumen de una sustancia es igual al cambio de volumen del agua cuando el objeto en cuestión se sumerge en el agua.
Dado que el volumen original del agua = 50 ml
Volumen de agua después de sumergir el objeto = 120 ml
Masa del objeto = 150 g
Ahora,
Densidad = masa / volumen
Densidad = 150g / 120-50 ml
Densidad = 150/70 ml
Densidad = 2,14 g / ml
Which one is a single replacement reaction? (Whoever gets it correct first I’ll mark)

Answers
The equation that represents a single replacement reaction given the various options is 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
What is a single replacement reaction?A single replacement reaction, also known as single displacement reaction is a reaction in which elements higher in the electro-chemical series displace or replace elements lower in the electro-chemical series displace from a solution.
The following example illustrates single replacement reaction:
A + BC -> AC + B
From the above reaction, we can see that A has replace/displace B to from AC.
With the above information, we can determine the equation that represents single replacement reaction. Details below:
Equation from the questions:
2Al + 3Cl₂ -> 2AlCl₃2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g)2AlCl₃(aq) -> 2Al + 3Cl₂ AlCl₃ + 3KOH -> Al(OH)₃ + 3KClFrom the above, we can see that only 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) conform to single replacement reaction.
Thus, the correct answer to the question is: 2Al(s) + 6HCl -> 2AlCl₃(aq) + 3H₂O(g) (option 2)
Learn more about single replacement reaction:
https://brainly.com/question/29662825
#SPJ1
radioactive materials can sometimes be used to determine the:
Answers
Radioactive materials can sometimes be used to determine various aspects, such as age, composition, or presence of certain substances.
Radioactive isotopes, through their decay processes, emit radiation that can be detected and measured. This property allows scientists to use them in techniques like radiometric dating, radiography, or tracer studies, which help determine the age of objects, analyze the composition of materials, or track the movement of substances in biological or industrial processes.
The use of radioactive materials in scientific research and applications provides valuable tools for studying and understanding a wide range of phenomena. From archaeological dating to medical imaging, radioactive materials have proven to be versatile and essential in numerous fields, enabling us to gain insights into the natural world and improve various aspects of human life.
To know more about Radioactive materials click here:
https://brainly.com/question/3542572
#SPJ11
N2(g) + 3H2(g) → 2NH3(g)
How many moles of N2 will react with 1.5 moles of H2?
a.1.5 mol
b. 1.0 mol
c. 0.5 mol
d. 2.0 mol
Answers
0.5 moles of N₂ will react with 1.5 moles of H₂.
N₂(g) + 3H₂ (g) -> 2NH₃(g)
From the above balanced equation
Three (3) moles of H₂ will reacts with 1 mole of N₂
1 moles of H₂(Hydrogen) will reacts with 1 / 3 moles of N₂(Nitrogen)
1.5 moles of H₂ requires 1 / 3 x 1.5 = 1 / 2 mole = 0.5 moles
Nitrogen is the chemical element having symbol N and its atomic number will be 7. Nitrogen is a nonmetal and the lightest member of the group 15 in the periodic table, often known as the pnictogens.
Hydrogen is the chemical element having symbol H and its atomic number will be 1. Hydrogen is the lightest element. At standard conditions hydrogen is a gas of the diatomic molecules having formula H₂. It is a colorless, tasteless, odorless, non-toxic, and highly combustible.
To know more about moles here
https://brainly.com/question/26416088
#SPJ4
where are class ii mhc found, and what cells recognize them?
Answers
Class II MHC molecules are important for the initiation of an immune response to extracellular pathogens, while class
I MHC molecules are important for the immune response to intracellular pathogens.
Major histocompatibility complex (MHC) molecules play an important role in the immune response. They are cell-surface proteins present on antigen-presenting cells (APCs), which function to bind and present antigenic peptides to T-cells. MHC molecules are classified into two types: class I and class II. Both classes have different roles and are present in different cells. Class I MHC molecules are present on the surface of all nucleated cells, while class II MHC molecules are only present on the surface of professional antigen-presenting cells such as dendritic cells, macrophages, and B cells.Cells recognize Class II MHC molecules are recognized by helper T-cells. Helper T-cells are a type of T-cell that recognize and bind to class II MHC molecules on the surface of antigen-presenting cells. This binding results in the activation of the helper T-cell, which then initiates an immune response against the antigen that is presented on the surface of the APCs. Class II MHC molecules are important for the initiation of an immune response to extracellular pathogens, while
class I MHC molecules are important for the immune response to intracellular pathogens.
To know more about molecules visit:
https://brainly.com/question/32298217
#SPJ11
The element s, q and r. Electronegativity of s is 6, that of q is 3.64 and r is 3.0 State with reasons i) Two elements that can form ionic bond. ii) Two elements that can form polar covalent bond. iii) Two elements that can form non-polar covalent bond.
Answers
i) Either Q or R could potentially form an ionic bond with S because the element S has the highest electronegativity (6), while the other two elements have lower electronegativity values.
ii)
S and Q are more likely to form a polar covalent bond because the electronegativity difference between S and Q (6 - 3.64 = 2.36) is greater than the electronegativity difference between S and R (6 - 3 = 3).
iii)
The electronegativity difference between Q and R (3.64 - 3 = 0.64) is relatively small, indicating that they are more likely to form a non-polar covalent bond.
What happens in polar covalent bond?In a polar covalent bond, the electrons are shared unequally between the two atoms, thus creating a partial positive charge on one atom and a partial negative charge on the other.
The greater the difference in electronegativity between the two atoms, the more polar the bond will be.
Learn more about polar covalent bond, at: https://brainly.com/question/3978603
#SPJ1
Determine the pore-water pressure (kn/m^2) at all locations where piezometers a through e have been installed. (note: unit weight of water = 9.81 kn/m^3.)
Answers
To determine the pore-water pressure (kn/m^2) at the locations where piezometers a through e have been installed, we need to use the unit weight of water, which is 9.81 kn/m^3.
Since the unit weight of water is given, we can calculate the pore-water pressure using the following equation: Pore-water pressure (kn/m^2) = Unit weight of water (kn/m^3) * Depth of piezometer (m) Unfortunately, the depths of piezometers a through e have not been provided in the question. Without this information, it is not possible to calculate the pore-water pressure at these specific locations. Given that the unit weight of water is provided as 9.81 kN/m³, we can convert the pressure head to kN/m² using the following formula: Pore-water pressure (kN/m²) = Pressure head (m) x Unit weight of water (kN/m³) Without specific readings or measurements from the piezometers, it is not possible to calculate the pore-water pressure at each location accurately.
The readings from the piezometers provide the necessary information about the water pressure at those specific points.
Read more about Pressure here;https://brainly.com/question/28012687
#SPJ11
The results of photosynthesis as glucose and oxygen
A) Photosynthesis
B)Products
C)Chloroplasts
D)Glucose
Answers
Answer:
Products
Explanation:
what does the chemical formula Ba(HCO3)2 tell us about number of atoms of each element present
Answers
Answer: The chemical formula is Ch(HCO3)2 and Cn(HO3)2
Explanation:
There is 1 atom of Ba, 2 atoms of hydrogen, 2 atoms of carbon, and 6 atoms of oxygen.
What are molecules?The smallest particle of a substance has all of the physical and chemical properties of that substance.
The chemical formula Ba(HCO3)2 tells us about the number of atoms of each element present as follows:
1 atom of Ba
2 atoms of hydrogen
2 atoms of carbon
6 atoms of oxygen.
Learn more about molecules here:
brainly.com/question/14130817
#SPJ2
this question is giving me a hard time

Answers
Answer:
options 3rd is the correct answer
what type of liquid is mercury
Answers
Answer:
Elemental mercury is the most common form. It is a metallic, silvery liquid ( also referred to as quicksilver) that is processed from an ore called cinnabar. It readily breaks into droplets and easily vaporizes at room temperature into an odorless, colorless vapor that can easily be inhaled.
Explanation:
Name the following covalent bond Ch4
Answers
Answer:
sp³-s sigma bond.
Hope that was the answer
At standard temperature and pressure, a given sample of water vapor occupies a volume of 2.80 L. How many hydrogen atoms are present in the container?
Answers
Explanation:
To determine the number of hydrogen atoms in the container, we need to know the number of water molecules present in the container.
At standard temperature and pressure (STP), which is defined as a temperature of 273.15 K and a pressure of 1 atmosphere (atm), one mole of any gas occupies a volume of 22.4 liters. Therefore, the number of moles of water vapor present in the container can be calculated as:
n = V/22.4
where V is the volume of the container in liters. Substituting the given value, we get:
n = 2.80/22.4 = 0.125
So, there are 0.125 moles of water vapor in the container.
Now, to determine the number of hydrogen atoms present in the container, we need to know the number of water molecules in the container, since each water molecule contains two hydrogen atoms. The number of water molecules can be calculated as:
N = n * N_A
where N_A is Avogadro's number, which is equal to 6.022 x 10^23 molecules per mole. Substituting the values, we get:
N = 0.125 * 6.022 x 10^23 = 7.528 x 10^22
So, there are 7.528 x 10^22 water molecules in the container, and since each water molecule contains 2 hydrogen atoms, the total number of hydrogen atoms in the container is:
2 * N = 2 * 7.528 x 10^22 = 1.506 x 10^23
Therefore, there are 1.506 x 10^23 hydrogen atoms present in the container.
At what position(s) will electrophilic aromatic substitution occur for the following compound?bromobenzene
Answers
In bromobenzene, the bromine atom is an electron-withdrawing group that deactivates the ring towards electrophilic substitution reactions. However, since the bromine atom is ortho-para directing, it directs incoming electrophiles to the ortho and para positions relative to itself.
Therefore, electrophilic aromatic substitution can occur at either the ortho or para positions in bromobenzene, and the meta position is less favored due to the deactivating nature of the bromine atom.
The reaction could be summarized as follows:
+ E+ + HX
| |
Ar-Br + E+ → Ar-E + HBr
| |
+ E+ + HX
where Ar represents the aromatic ring, Br represents the bromine atom, E+ represents the electrophile, and HX represents the acid catalyst.
learn more about electrophilic here:
https://brainly.com/question/29454942
#SPJ4
what is the molar solubility of ca3(po4)2? (ksp of ca3(po4)2 = 2.0×10−29)
Answers
The molar solubility of Ca₃(PO₄)₂ is 4.4 × 10⁻¹⁰ M, using the Ksp value of 2.0 x 10⁻²⁹. This means that only a small amount of the compound will dissolve in solution.
The molar solubility of Ca₃(PO₄)₂ can be calculated using its solubility product constant (Ksp) which is given as 2.0 × 10⁻²⁹.
The solubility product expression for Ca₃(PO₄)₂ is:
Ca₃(PO₄)₂ ⇌ 3Ca²⁺ + 2PO₄²⁻
Ksp = [Ca²⁺]³ [PO₄⁻²]²
Let x be the molar solubility of Ca₃(PO₄)₂. Then at equilibrium, the concentration of Ca²⁺ and PO₄²⁻ ions will be 3x and 2x, respectively.
Substituting these values into the solubility product expression and solving for x, we get:
Ksp = (3x)³ (2x)²
2.0 × 10⁻²⁹ = 108x⁵
x = (2.0 × 10⁻²⁹ / 108)^(1/5)
x = 4.4 × 10⁻¹⁰ M
Therefore, the molar solubility of Ca₃(PO₄)₂ is 4.4 × 10⁻¹⁰ M.
To know more about the Ca₃(PO₄)₂ refer here :
https://brainly.com/question/31435448#
#SPJ11