Which of the following has the ability to cause motion and create change?
Energy
Space
Sound
Mass
Answers
Answer: Energy has the ability to cause motion and create change.
Related Questions
We can prove a theory to be correct by performing the right experiment. False or true
Answers
Answer:
true
Explanation:
because if you find the right answer that proves the theory to be correct.
Structural Formulas for Covalent Molecules
Is anyone good at doing these because I am so confused
1) C2H4
2) C3Cl8
3) SCl2
4) HClO2
5) HNO2
6) H2SeO3
7) H2S2
8) C2H6O2
9) C2H7N
10) C2NH3
11) C2PSH3
12) CH2O2PN
13) C2H2S
14) CH2O
15) N2H2
Answers
Answer:
CFJA17THZ561GJA561GH68N461DG56BSJ57
HOPE IT HELPS THANK ME LATER.
A(n)________ can change shape and volume.
Answers
Answer:
gas and liquid
Explanation:
right?
If 400cm³ of Q was collected at 250°c and 1.20×10³nm², calculate the volume it would occupy at STP
Answers
Answer:
2.481cm³
Explanation:
this is general has equation so the formula is
P1V1/T1 = P2V2/T2
At STP, pressure is 1.01*10⁵ and temperature is 273K
((1.20*10³)400)/(250+273) = ((1.01*10⁵)V2/(273)
V2 = 2.481cm³
How does the temperature change when a layer of glass is added?
Answers
Answer:
thermal shock
Explanation:
the temperatures inside the glass jar should have continued to increase over time. Internal stresses due to uneven heating. This is also known as “thermal shock”.
In general, the thicker the glass, the more prone it will be to breaking due to the immediate differences in temperature across the thickness of glass.
Borosilicate glass is more tolerant of this, as it has a higher elasticity than standard silicon glass.
You may also note that laboratory test tubes and flasks are made with thinner walls, and of borosilicate glass, when designated for heating.
How do you correctly put the number 0.00000000008759 in scientific notation with 3 sig figs?
• 8.76 x 10-11
O 87.6 x 10-11
O 8.759 x 10-11
O 8.759 x 1011
Answers
Answer:
8.759×10-11
Explanation:
it may be to sure try to convert the answer
At what temperature will water change from a liquid to a solid?
Answers
Answer:
32 degrees Fahrenheit
Answer:
32f or 0c
Explanation:
32 ferenhiet or 0 celsius
What neutral atom has a nuclear charge of +9, and a nuclear mass of 19 amu? How many neutrons does it have. How many electrons does it have ?
Answers
The neutral atom with a nuclear charge of +9 and a nuclear mass of 19 amu is fluorine-19. It has 10 neutrons and 9 electrons.
The atomic number of an element is equal to the number of protons in its nucleus. Therefore, if an atom has a nuclear charge of +9, it means it has 9 protons. The nuclear mass of an atom is the sum of its protons and neutrons. Since we know the nuclear mass of this atom is 19 amu, and it has 9 protons, it must have 10 neutrons (19 - 9 = 10).
To determine the number of electrons, we need to know the charge of the atom. Since it is a neutral atom, it has no net charge. This means the number of electrons must be equal to the number of protons, which is 9.
Therefore, the identity of the neutral atom is fluorine-19, with 9 protons, 10 neutrons, and 9 electrons.
To know more about protons refer here:
https://brainly.com/question/30895149#
#SPJ11
Epinephrine (adrenaline) is a hormone secreted into the bloodstream in times of stress. It contains 59.0% C, 7.15% H, 26.20% O, and 7.65% N and has a molar mass of 183 g/mol. What is its molecular formula
Answers
Answer:
C9H13O3N
Explanation:
Take the atomic mass of C=12.0, H=1.0, O=16.0 and N=14.0.
We can draw a chart (please view this on desktop so to avoid spacing errors):
Let the mass of Epinephrine be 100g.
C H O N
mass(g) : 59 7.15 26.2 7.65
no. of moles : 59/12 =4.9167 7.15/1 = 7.15 26.2/16=1.6375 7.65/14=0.5464
(n.o.m. = mass/molar mass)
Ratio: 4.9167/0.5464 =9 7.15/0.5464=13 1.6375= 3 0.5464/0.5464 =1
(divide the n.o.m. by the smallest n.o.m., which is 0.5464 in this case, take the whole numbers)
So, the empirical formula will be: C9H13O3N
But this is still not yet the molecular formula. We have to ensure the molar mass is 183g/mol. Multiply the empirical formula by n.
So, let the molecular formula of Epinephrine be (C9H13O3N)n.
12x9n + 1x13n + 16x3n + 14n = 183
108n + 13n + 48n + 14n = 183
183 n = 183.
n = 1
Hence, the molecular formula is C9H13O3N.
Branched-chain amino acids (bcaas), commonly known as a supplement to support recovery and stimulate muscle growth, may also serve to:_____.
Answers
BCAA supplements are commonly taken to boost muscle growth and enhance exercise performance.
They may also help with weight loss and reducing fatigue after exercise. This article contains all the most important information about branched-chain amino acids and their benefits.
What are purposes of Branched-chain amino acids (bcaas) ?Leucine, isoleucine, and valine are the three essential amino acids that make up the branched-chain amino acids (BCAAs).Since they are vital, your body cannot create them on its own and you must get them through food.BCAA supplements have been demonstrated to increase muscular mass, lessen muscle pain, and reduce muscle fatigue.They have also been used successfully in hospitals to lessen liver disease symptoms and stop or slow down muscle loss.However, since the majority of people consume enough BCAAs from their diets, taking supplements is unlikely to provide any further advantages.To view more about amino acids, refer to:
https://brainly.com/question/15969022
#SPJ4
Fill in the blank: The units Hertz (Hz) is used to express _________________________. options: wave period wavelength amplitude frequency
Answers
Answer:
Hertz is the rate at which current changes direction. SO frenquency
Explanation:
Tornadoes can negatively affect an ecosystem when
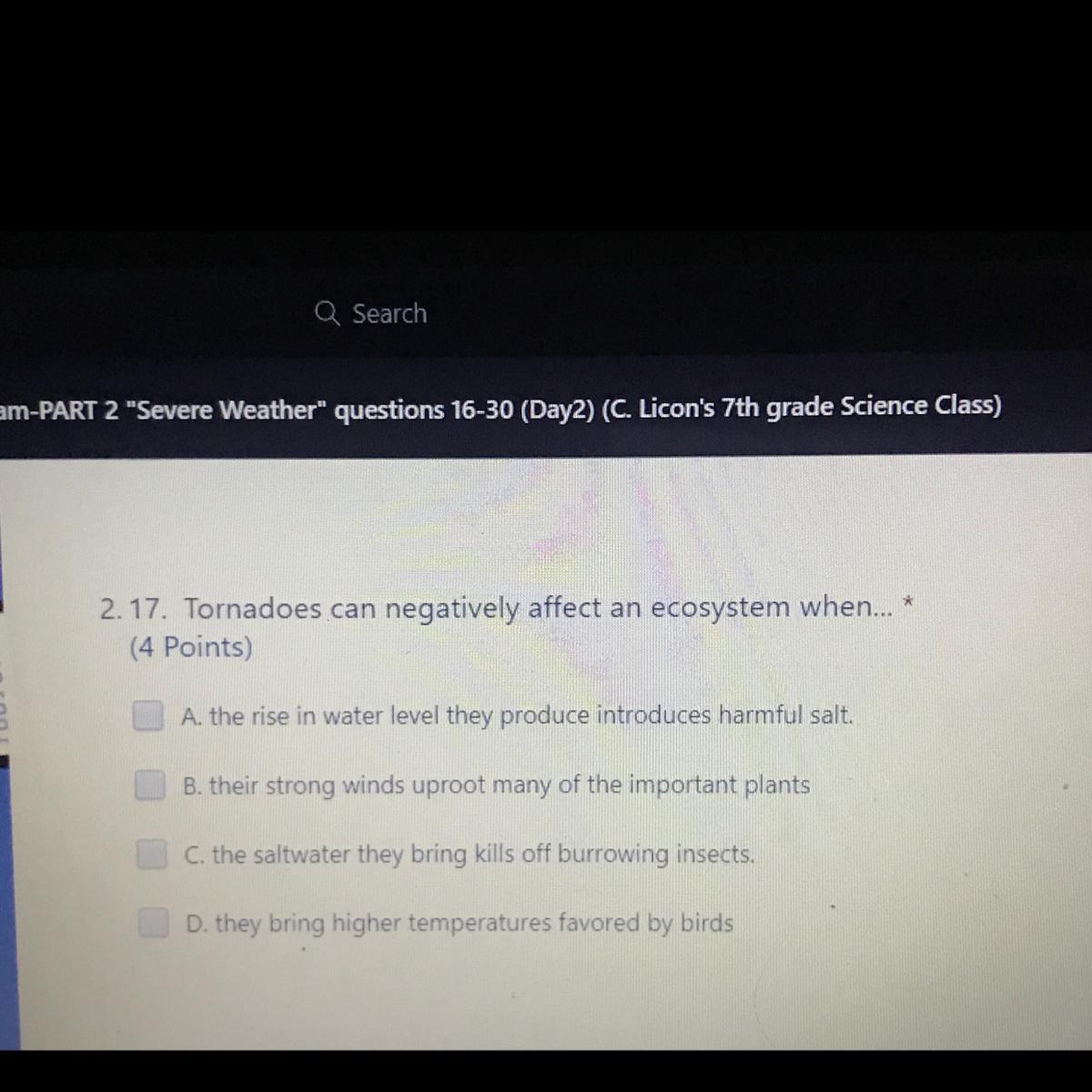
Answers
Answer:
b, their strong winds uproot many of the important plants
Explanation:
What would happen if a small amount of base were added to a buffered solution?
OA. The pH would remain about the same.
OB. The pH would remain neutral.
OC. The pH would decrease.
OD. The pH would increase.
Answers
Please help me, I will give you brainlist

Answers
Answer:
ʟᴇᴛᴛᴇʀ: ᴄ.
Explanation:
ɪʙᴀ ᴀɴɢ ᴇxᴘʟᴀɴᴀᴛɪᴏɴ sᴀ ʟᴀʜᴀᴛ ɴɢ ᴀɴsᴡᴇʀ
What is the mass, in grams, of 1.33 mol of water, H2O? Express the mass in grams to three significant figures.
Answers
Explanation:
First find the mass of 1 mole of water (Molecular mass)
\( H_{2} O = 2H + O \)
\(
= 2(1) + 32 \)
\(
= 34 \: grams
\)
Hence 1 mole of water is 34 g
Compare
1 mole = 34 g
1.33 mole = x g
\( \frac{x}{34} = \frac{1.33}{1} \)
\(
x = 1.33 \times 34 \)
\(
\color{blue}{\boxed{x = 45.2}} \: \: \: \: to \: 3 \: s.f.
\)
Therefore 1.33 moles of water is 45.2 grams
The mass of the substances can be given by the molar mass and the moles of the substances. The mass of 1.33 mole of water is expressed in grams as, 23.94 grams.
What is mass?The mass has been defined as the product of the moles of the substance and the molar mass in grams per mole. The mass is given in grams and has a formula,
Moles = mass ÷ molar mass
Mass = molar mass × moles
Given,
Moles = 1.33 moles
Molar mass of water (H₂O) is calculated as = 2 (1) + 16= 18 grams per mole
Using the formula of mole, mass is calculated as:
Mass = molar mass × moles
mass = 1.33 × 18
= 23.94 grams
The mass of the substance can be calculated if the value of the molar mass and the moles of the substance has been known. The molar mass of the substance can be calculated by adding the mass of the individual masses of the element in a molecule.
Therefore, 1.33 mol of water contains 23.94 grams.
Learn more about mass, here:
https://brainly.com/question/20970268
#SPJ2
which gives the set of particles that experience stronger attration and explains why

Answers
The set B gives the set of particles that experience stronger attraction because the charges are greater. Therefore, option B is correct.
What do you mean by the charge ?Electric charge is the physical property of matter. Charges makes to experience a force when placed in an electromagnetic field.
Positive and negative electric charges are the two types of charges commonly transported by charge carriers, protons and electrons. Energy is make up by the movement of charges.
In set B there are +4 and -4 two charges are present so, they give stronger attraction that set A.
Thus, option B is correct.
To learn more about the charge, follow the link;
https://brainly.com/question/3412043
#SPJ1
what is waves is energy passing through the medium of water? true or false
Answers
i have no idea what you said but for the sake of god its true
using equation of reaction mention 4 methods of preparation of hydrogen
Answers
Explanation:
1. Displacement of hydrogen from water by using metal
2. Electrolysis of water using the apparatus known as the HOFMAN VOLTAMETER
3. Cracking of petroleum
4. Action of steam on hot coke
Look at the activity series and select which two of the following reactions would happen on their own. (Remember, if the lone element is more active than the metal in the compound, the lone element will react and replace the metal in the compound.) Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (Al)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
A.
2Li + ZnBr2 ->2LiBr + Zn
B.
Al + 3LiCl ->AlCl3 + 3Li
C.
Sn + ZnSe ->SnSe + Zn
D.
3Ca + Al2O3 ->2Al + 3CaO
Answers
Answer:
2Li + ZnBr2 ->2LiBr + Zn
3Ca + Al2O3 ->2Al + 3CaO
Explanation:
Spontaneous reactions are reactions that can happen on their own. For a spontaneous reaction to occur, a metal that is higher in the activity series must displace a metal that is lower in the activity series from its solution and not vice versa.
If we look at the two reactions selected in the answer, lithium is above zinc in the activity series and calcium is above aluminum in the activity series hence the two reactions occur spontaneously.
c. Convert 1.05x107 milliliters to kiloliters (L = liter)
Answers
Answer:
10.5 kl
Explanation:
\(1.05*10^7ml\\=\frac{1.05*10^7}{10^3}l\ [1 ml= 1/1000 l]\\=1.05*10^4\\=10500 l\\=\frac{10500}{1000} l\ [1l=1/1000kl]\\=10.5 kl\)
Hope this helps. :)
At 25 celcius, what is the keq for a reaction where delta h=-6.3kj/mol and delta =70.5
Answers
To calculate the equilibrium constant (K_eq) at 25 degrees Celsius for a reaction with ΔH = -6.3 kJ/mol and ΔS = 70.5 J/(mol·K), we can use the equation:
ΔG = ΔH - TΔS
where ΔG is the change in Gibbs free energy, ΔH is the change in enthalpy, ΔS is the change in entropy, and T is the temperature in Kelvin.
At equilibrium, ΔG = 0, so we can rearrange the equation to solve for the equilibrium constant:
ΔG = -RT ln(K_eq)
Since ΔG = 0 at equilibrium, we have:
0 = -RT ln(K_eq)
R is the gas constant (8.314 J/(mol·K)) and T is the temperature in Kelvin (25 + 273.15 = 298.15 K). By substituting these values into the equation, we can solve for ln(K_eq) and then calculate K_eq.
Explanation:
In thermodynamics, the equilibrium constant (K_eq) relates the concentrations of products and reactants at equilibrium. It is a measure of the extent to which a reaction proceeds in the forward or reverse direction. The equilibrium constant can be determined using the Gibbs free energy change (ΔG), which combines the enthalpy change (ΔH) and the entropy change (ΔS) of the reaction.
In this case, we are given ΔH = -6.3 kJ/mol and ΔS = 70.5 J/(mol·K) for the reaction. By substituting these values into the equation ΔG = ΔH - TΔS, we can find the value of ΔG at the given temperature of 25 degrees Celsius (or 298.15 K).
Since ΔG is zero at equilibrium, we can rearrange the equation to -RT ln(K_eq) = 0 and solve for ln(K_eq). By substituting the appropriate values for R (gas constant) and T (temperature), we can calculate ln(K_eq). Finally, taking the exponential of ln(K_eq) gives us the equilibrium constant K_eq for the reaction at 25 degrees Celsius.
Learn more about equilibrium constant here :
brainly.com/question/28559466
#SPJ11
Round each of the following measurements to the number of significant figures
indicated.
67,029 to three significant figures
0.15 to one significant figure
52.8005 to five significant figures
3.17497 to three significant figures
Answers
Answer:
a) 67000 b) 0.20 c) 52.8010 d) 3.17000
Explanation:
your welcome :)
a) 67000 b) 0.20 c) 52.8010 d) 3.17000 are the answers respectively.
What is measurement?Distance is a physical quantity which is measured in meter. If a man travel then it is measured in meters that defines that how much he travel from one point to another point for all these type of things we use to measure distance.
Centimeter is also a physical quantity which is use to measure distance. 1 centimeter is equal to 0.01 meter and 1 centimeter is equal to 0.00001 kilometer.the kilometer is large quantity to measure distance while centimeter is smaller quantity to measure distance.
Kilometer is also a physical quantity which use to measure the distance 1 kilometer is equal to 1000 mtr and 1 kilometer is equal to 100000 centimeter.
For above conclusion, we easily states that the kilometer is large quantity to measure distance while centimeter is smaller quantity to measure distance. Centimeter is also a physical quantity which is use to measure distance. 1 centimeter is equal to 0.01 meter and 1 centimeter is equal to 0.00001 kilometer.
Therefore, a) 67000 b) 0.20 c) 52.8010 d) 3.17000 are the answers respectively.
Learn more about measurement here:
https://brainly.com/question/2107310
#SPJ2
Calculate the maximum β - energy in the decay of
14
C to
14
N. The mass of
14
C is 14.003241u; the mass of
14
N is 14.003074u
Answers
Question id : 33544103
Answer:
To calculate the maximum β-energy in the decay of carbon-14 (14C) to nitrogen-14 (14N), we need to use the mass difference between the initial and final nuclei. The β-decay process involves the conversion of a neutron into a proton, emitting a β-particle (electron) and an electron antineutrino.
The mass difference (∆m) between 14C and 14N is given by:
∆m = mass(14C) - mass(14N)
= 14.003241u - 14.003074u
= 0.000167u
The β-energy (Eβ) is related to the mass difference (∆m) by Einstein's mass-energy equivalence equation: E = ∆mc^2, where c is the speed of light.
Eβ = ∆m * c^2
Now, we need to convert the mass difference (∆m) from unified atomic mass units (u) to kilograms (kg) before using it in the equation. The conversion factor is approximately 1.66053906660 × 10^−27 kg/u.
∆m_kg = ∆m * (1.66053906660 × 10^−27 kg/u)
Next, we'll calculate the β-energy by substituting the values into the equation:
Eβ = ∆m_kg * c^2
The speed of light (c) is approximately 2.998 × 10^8 m/s.
Eβ = ∆m_kg * (2.998 × 10^8 m/s)^2
Let's perform the calculations:
∆m_kg = 0.000167u * (1.66053906660 × 10^−27 kg/u)
≈ 2.77273363362 × 10^−29 kg
Eβ = (2.77273363362 × 10^−29 kg) * (2.998 × 10^8 m/s)^2
≈ 2.49344368869 × 10^−13 J
The maximum β-energy in the decay of 14C to 14N is approximately 2.493 × 10^−13 Joules.
To know more about β - energy visit:
https://brainly.com/question/33544103
#SPJ11
Which of the essential nutrients is listed in the course as actually a group of micronutrients?
Answers
Vitamins and Minerals
Micronutrients, often known as vitamins and minerals, are essential for healthy growth, illness prevention, and overall well-being. Micronutrients, apart from vitamin D, are not generated by the system and must be obtained from food. Even though humans only require trace levels of micronutrients, ingesting the required quantity is critical. Micronutrient deficits can be life-threatening. At least the majority of all children under the age of five suffers from deficiencies of vitamins and minerals.
Despite the fact that humans only require modest levels of micronutrients, it's crucial to eat the suggested quantity. Devastating repercussions can result from micronutrient deficits. Around the world, at least 50% of children under the age of five have vitamin and mineral deficiencies.
To know more about micronutrients refer to https://brainly.com/question/6974286
#SPJ4
What are some of the different substances that make up a pizza?
What substances make up water?
Answers
Answer:
Explanation:
What kind of ridiculous chemistry question asks you the composition of a pizza?
You could technically write anything along the lines of cheese, bread, tomato-paste, unless the question specifically asks for chemical details.
Water is made from a bond of Hydrogen and Oxygen atoms.
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2?
Answers
If you started with 1.5 moles of H2 at STP, you would have approximately 33.6 liters of volume of hydrogen (H₂) gas.
What is the volume of the hydrogen gas at STP?
To determine the number of liters of H2 you have, we need to consider the conditions under which the gas is being held (i.e. temperature and pressure), as well as the molar volume of H2 at those conditions.
At standard temperature and pressure (STP), which is 0°C (273.15 K) and 1 atm (101.325 kPa), the molar volume of any ideal gas is approximately 22.4 L/mol.
Therefore, at STP, 1.5 moles of H₂ would occupy approximately:
V = n x Vm = 1.5 mol x 22.4 L/mol = 33.6 L
Learn more about volume of gas here: https://brainly.com/question/25736513
#SPJ1
The complete question is below:
HOW MANY LITERS OF H2 DO YOU HAVE IF YOU START WITH 1.5 MOLES OF H2? (assume STP condition)
what is the central atom? enter its chemical symbol. how many lone pairs are around the central atom? what is the ideal angle between the carbon-chlorine bonds? compared to the ideal angle, you would expect the actual angle between the carbon-chlorine bonds to be ...
Answers
The central atom refers to the atom in a molecule that is bonded to multiple other atoms. Its chemical symbol depends on the specific molecule being discussed. The number of lone pairs around the central atom varies depending on the molecule's structure.
The central atom is the atom in a molecule that is bonded to multiple other atoms. Its chemical symbol varies depending on the specific molecule under consideration. The number of lone pairs surrounding the central atom depends on the molecular structure and the distribution of electrons. For example, in the molecule carbon tetrachloride (CCl4), the central atom is carbon (C), and there are no lone pairs around it.
According to the theory of VSEPR (Valence Shell Electron Pair Repulsion), the ideal angle between the carbon-chlorine bonds in carbon tetrachloride is 109.5 degrees. This angle arises from the repulsion between electron pairs around the central carbon atom. However, in practice, the actual angle between the carbon-chlorine bonds may deviate slightly from the ideal angle due to various factors such as steric hindrance, molecular strain, and the presence of other atoms or functional groups in the molecule. The ideal angle between carbon-chlorine bonds in a molecule such as carbon tetrachloride (CCl4) is 109.5 degrees, according to the theory of VSEPR (Valence Shell Electron Pair Repulsion). In reality, due to various factors, the actual angle between the carbon-chlorine bonds may deviate slightly from the ideal angle.
Learn more about atom here:
https://brainly.com/question/26952570
#SPJ11
Consider the reaction of C6H6 + Br2 -------> C6H5Br + HBr What is the theoretical yield of C6H5Br if 42.1 g of C6H6 react with 67.5 g of Br2? Answer to 3 sig figs. (1 decimal place)
Answers
The theoretical yield of C6H5Br is 66.3 g (to 3 sig figs) when 42.1 g of C6H6 reacts with 67.5 g of Br2 after using the stoichiometry of the balanced equation.
To determine the theoretical yield of C6H5Br, we need to calculate the limiting reactant and use the stoichiometry of the balanced equation.
First, we need to find the moles of each reactant using their respective molar masses:
Molar mass of C6H6 (benzene) = 78.11 g/mol
Molar mass of Br2 = 159.81 g/mol
Moles of C6H6 = 42.1 g / 78.11 g/mol = 0.538 mol (to 3 sig figs)
Moles of Br2 = 67.5 g / 159.81 g/mol = 0.422 mol (to 3 sig figs)
Now we can compare the mole ratios between C6H6 and Br2 in the balanced equation:
C6H6 + Br2 → C6H5Br + HBr
From the equation, we see that the stoichiometric ratio is 1:1 between C6H6 and C6H5Br. Therefore, the limiting reactant is the one with fewer moles, which is Br2 in this case.
Now we can calculate the theoretical yield of C6H5Br using the mole ratio and the molar mass of C6H5Br:
Molar mass of C6H5Br = 157.02 g/mol
Theoretical yield of C6H5Br = moles of limiting reactant (Br2) × molar mass of C6H5Br
= 0.422 mol × 157.02 g/mol
= 66.3 g (to 3 sig figs)
To know more about stoichiometry, please click on:
https://brainly.com/question/28780091
#SPJ11
what are the factors affecting gravity?
Answers
Gravity, as a fundamental force of nature, is influenced by several factors. The following are some of the key factors affecting gravity:
Mass: The most significant factor affecting gravity is the mass of the objects involved. According to Newton's law of universal gravitation, the gravitational force between two objects is directly proportional to the product of their masses. Greater mass leads to a stronger gravitational force.Distance: The distance between two objects also plays a crucial role in the strength of gravity. According to the inverse square law, the gravitational force decreases as the distance between objects increases. As objects move farther apart, the gravitational attraction between them weakens.Gravitational Constant: The gravitational constant, denoted by G, is a fundamental constant in physics that determines the strength of the gravitational force. It is a universal constant and does not change, affecting the overall magnitude of gravity.Shape and Distribution of Mass: The distribution of mass within an object can influence the gravitational field it generates. Objects with a more compact and concentrated mass distribution will have a stronger gravitational pull compared to those with a more spread-out mass distribution.External Influences: Gravity can be influenced by external factors such as nearby celestial bodies or the presence of other forces. For example, the gravitational interaction between the Earth and the Moon affects tides on Earth's surface.what physical properties could be used to separate iron filings from table salt
Answers
Answer:
Iron is magnetic and Salt is non magnetic
So Salt dissolves in water and Iron doesn't