Which of the following is NOT a factor in how much voltage is produced by a
generator?
How fast the magnet in the generator is moving through the coiled wire
How many amps are run through the electromagnet of the generator
How strong the magnet in the generator actually is
How many times the wire is coiled in the generator....
Answers
Answer:
How fast the magnet in the generator is moving through the coiled wire
Explanation:
Another name for an electric generator is the electric motor. It is a device that is used to convert mechanical energy into electrical energy.
It comprises of a rectangular coil of insulated wire called armature which rotates about a fixed axis. Powerful magnets provides the required magnetic field. A commutator made of split ring copper and two carbon brushes are attached to it.
The voltage produced by the electric motor depends on;
i) The strength of the magnetic field.
ii) The number of turns of the coil
iii) The current across the armature
Related Questions
The combustion of octane, C8H18, proceeds according to the reaction shown.
2C8H18(l)+25O2(g)⟶16CO2(g)+18H2O(l)
If 354 mol of octane combusts, what volume of carbon dioxide is produced at 15.0 ∘C
and 0.995 atm?
Answers
The concept ideal gas equation is used here to determine the volume of the carbondioxide. Combustion reactions are generally highly exothermic reactions. The volume of CO₂ is
A combustion is a chemical reaction in which a fuel undergoes oxidation as a result of the reaction with an oxidizing agent which causes the release of energy in the form of heat.
15.0 °C = 288 K
The ideal gas equation is:
PV = nRT
V = nRT / P
V = 354 × 0.0821 × 288 / 0.995 = 8412.3 L
To know more about combustion, visit;
https://brainly.com/question/14335621
#SPJ1
C6H12O6 + 602 → 6CO2 + 6H₂O
The most efficient ratio is
1 C6H12O6 6 02.
Which set of reactants will be the most
efficient (least wasteful of materials) for
the reaction?
A. 1.0 mol C6H12O6 and 3.0 mol O₂
B. 1.5 mol C6H₁2O6 and 3.0 mol O₂
C. 3.0 mol C6H₁2O6 and 6.0 mol O₂
D. 0.5 mol C6H₁2O6 and 3.0 mol O₂
Answers
Answer:
D
Explanation:
The ratio of C6H12O6 (which will be referred to as "the carb") to oxygen is 1 to 6, so if we find an answer which has the same ratio, it should be chosen. A is 1:3
B is even worse with a ratio of the carb to oxygen of 1:2
C is the same as B, 1:2
D has a ratio of the carb to oxygen of 1:6, which is what we are looking for.
Weather is not the same as climate. Which claim
identifies the main difference between these two concepts?
A. The main difference is how both are measured
B. Only weather includes information about the
temperature
C. Only climate includes information about
precipitation
O D. The main difference is the length of time over
which both are measured
Answers
Answer:
b. only weather includes information about the temperature
Which of the following is NOT correct about the mole concept?
1 point
1 mole of sodium atoms will ionize to produce 1 mole of sodium ions and 1 mole of
electrons
A mole is the number of carbon atoms in 12g of C-12 isotope.
A mole is the number of molecules in 22.4 dm3 of all gases at all temperatures and
pressures
A mole is the number of electrons that carry one Faraday of electricity
A mole is the number of molecules of water in 18 g of ice, water or water vapour
Answers
Answer:
a mole is the number of molecules in 22.4 dm³ for all gases at all temperatures and pressures
Based on this chart, which of the following statements describes sources of
energy in the United States?
Sources of Energy
Petroleum
3796
Other
91%
Natural Gas
24%
Renewable
Energy
7%
Coal
2396
*Nuclear
Electric Power
8%
A. Most energy comes from nuclear power and renewable sources.
B. Most energy comes from nonrenewable energy sources.
C. Most energy comes from sources in the solid phase.
D. Most energy comes from greenhouse gases.
Answers
Answer:
B. Most energy comes from non-renewable sources
Explanation:
A P E X
In United States Most energy comes from non-renewable energy sources, as petroleum coal and others has the highest consumption in the given chart
What is the energy source in US?In the given chart, the energy consumption in US is based on Petroleum, coal and others the highest consumption pattern as compared to other energy sources
Hence, most energy in the US is from non-renewable energy sources
Learn about renewable energy sources.
https://brainly.com/question/4038933
#SPJ2
What is the oxidation state of O in NO₂?
OA. +2
OB. -2
O C. O
OD. -4
Answers
Answer:
Let it be x
\({ \tt{ (oxidation \: state \: of \: nitrogen) + 2x = overall \: charge}} \\ { \tt{ - 4 - 2 x = 0}} \\ { \tt{2x = - 4}} \\ { \tt{x = - 2}}\)
produces pyruvate. the multienzyme complex catalyzes the oxidative of pyruvate to yield carbon dioxide and acetyl coa. the overall equation for
Answers
Pyruvate is a byproduct of glycolysis.The oxidative deacetylation of pyruvate to produce carbon dioxide and acetyl CoA is catalyzed by the multienzyme complex dopamine dehydrogenase complex (PDH complex).Pyruvate + CoA + NAD+ ----> Acetate CoA + NADH + H++ CO2. Acetyl CoA ———- Citric Acid Cycle is the general equation for the reaction.
How is acetyl CoA produced from pyruvate?Coenzyme A is joined with the oxidized two-carbon acetyl group to generate acetyl CoA.
What enzyme is in charge of turning pyruvate into acetyl CoA?The pyruvate dehydrogenase (PDH) enzyme, which is a component of the multienzyme PDC and is frequently described to as a "gatekeeper" in the oxidation of carbohydrates, catalyzes the physiologically irreversible conversion of pyruvate to acetyl-CoA.
To know more about yield carbon dioxide visit:
https://brainly.com/question/14995953
#SPJ4
Which has a different answer?
11 - 6 + 3
6 + 11 - 3
3 + 11 - 6
11 + 3 - 6
Answers
Therefore, 6 + 11 - 3 has different answer.
Answer:
The answer is B- 14
Explanation:
11-6+3= 5+3= 8
6 + 11 - 3= 17-3=14
3 + 11 - 6= 14-6=8
11 + 3 - 6= 14-6=8
Magnesium carbonatea n d hydrochloric acid react to produce salt, water and carbon
dioxide.
MgcO, + 2 HCt m MgCh + H,0 +CO. .
What is the volume of CO, produced when 21 g of magnesium carbonate reacts
completely with excess hydrochloric acid?
A 4 dma
B 8dm°
C 6dm D 2dm
Answers
Answer:
Explanation:
The balanced chemical equation for the reaction between magnesium carbonate and hydrochloric acid is:
MgCO3 + 2HCl → MgCl2 + H2O + CO2
From the equation, we can see that 1 mole of magnesium carbonate reacts with 2 moles of hydrochloric acid to produce 1 mole of carbon dioxide. The molar mass of magnesium carbonate is 84.3 g/mol, which means that 21 g of magnesium carbonate is equal to 0.25 moles (21/84.3). Therefore, 0.25 moles of magnesium carbonate will react with 0.5 moles of hydrochloric acid to produce 0.25 moles of carbon dioxide.
The volume of carbon dioxide can be calculated using the ideal gas law, which is PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature. Assuming standard temperature and pressure (STP) of 273 K and 1 atm, we can use the molar volume of a gas at STP, which is 22.4 L/mol, to calculate the volume of carbon dioxide produced.
V = n × 22.4 L/mol
V = 0.25 mol × 22.4 L/mol
V = 5.6 L
Therefore, the volume of carbon dioxide produced when 21 g of magnesium carbonate reacts completely with excess hydrochloric acid is 5.6 L. The answer is option A, 4 dm³, which is approximately equal to 5.6 L.
When a sample of a gas is heated at constant pressure, the average kinetic energy of its molecules —
Answers
When a sample of a gas is heated at constant pressure, the average kinetic energy of its molecules vibrate.
Thus, An object's kinetic energy is the type of energy that it has as a result of motion. It is described as the effort required to move a mass-determined body from rest to the indicated velocity and vibrate.
The body holds onto the kinetic energy it acquired during its acceleration until its speed changes. The body exerts the same amount of effort when slowing down from its current pace to a condition of rest.
Formally, kinetic energy is the second term in a Taylor expansion of a particle's relativistic energy and any term in a system's Lagrangian that includes a derivative with respect to time.
Thus, When a sample of a gas is heated at constant pressure, the average kinetic energy of its molecules vibrate.
Learn more about Kinetic energy, refer to the link:
https://brainly.com/question/999862
#SPJ1
Balance the following equationK3PO4 + HCl => KCl + H3PO4
Answers
1) Write the chemical equation.
\(K_3PO_4+HCl\rightarrow KCl+H_3PO_4\)List the elements (or polyatomic ions) in the reactants.
K: 3
P: 1
O: 4
H: 1
Cl: 1
List the elements (or polyatomic ions) in the reactants.
K: 1
P: 1
O: 4
H: 3
Cl: 1
2) Balance K.
\(K_3PO_4+HCl\rightarrow3KCl+H_3PO_4\)List the elements (or polyatomic ions) in the reactants.
K: 3
P: 1
O: 4
H: 1
Cl: 1
List the elements (or polyatomic ions) in the reactants.
K: 3
P: 1
O: 4
H: 3
Cl: 3
3) Balance Cl.
\(K_3PO_4+3HCl\rightarrow3KCl+H_3PO_4\)List the elements (or polyatomic ions) in the reactants.
K: 3
P: 1
O: 4
H: 3
Cl: 3
List the elements (or polyatomic ions) in the reactants.
K: 3
P: 1
O: 4
H: 3
Cl: 3
4) The balanced chemical equation.
\(K_3PO_4+3HCl\rightarrow3KCl+H_3PO_4\).
this woman is riding a bicycle down a hill at a constant speed and in a straight line.
Which change will increase the speed of the bicycle?
A. Added forces of 30 N up the hill and 20 N down the hill
B. Added forces of 30 N up the hill and 30 N down the hill
C. An added force of 20 N down the hill
D. An added force of 20 N to the side of the hill

Answers
Answer:
what is your zoom email
teĺlllll
In the chemical equation CH4 + O2 -> CO2 + 2H2O for every one mole of carbon dioxide you produced, how many moles of water would you have?
Answers
Answer: 2 moles
Explanation:
In the balanced equation, it can be seen that everything reacts in a 1:1 mol ratio except water (H2O) as determined by the coefficient. It is simple stoichiometry, 1 mol CO2 * 2 mol H2O/1 mol CO2 = 2 mol CO2. After doing the math, the CO2 cancels out, leaving how many moles of H2O there are.
PLEASE HELP!!!!!!!
In an experiment, the molar mass of the compound was determined to be 118.084 g/mol. What is the molecular formula of the compound?
Answers
Succinic acid most probably
C_4H_6O_4Lets see why?
The molar mass is given as 118.084g/mol
Decimal next place i.e 10th place contains 0 means it has OH bonds in most of the cases.
So it's a hydrocarbon and more closely an alcohol or aldehyde
Let's verify
4(12)+6(1)+16(4)64+48+670+58118g/molApproximately equal
What is the average atomic mass of 10 hydrogen -1 molecules?
Answers
Answer:
1.674 x 10^-23 grams
Explanation:
Hydrogen-1 is called Protium
wikipedia
atomic mass of Protium is 1.00794 amu
sciencedirectcom
atomic mass of 10 Protiums is 10.0794 amu
10.0794 amu in grams is
1.6737236x10^-23 grams
I need help I don’t understand this is hitting

Answers
Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Thus, They are additionally known as limiting reactants or limiting agents. A predetermined quantity of reactants are necessary for the reaction to be completed, according to the stoichiometry of chemical reactions.
In the aforementioned reaction, 2 moles of ammonia are created when 3 moles of hydrogen gas react with 1 mole of nitrogen gas.
In most cases, this reactant dictates when the reaction will end. The reaction stoichiometry can be used to determine the precise quantity of reactant that will be required to react with another element. The limiting agent is determined by the mole ratio rather than the mass of the reactants.
Thus, Reagents that are entirely consumed by a chemical reaction are known as limiting reagents.
Learn more about Chemical reaction, refer to the link:
https://brainly.com/question/22817140
#SPJ1
write the structural formula for 2-bromo-3-chloro-4,4-dimethylpentanal
Answers
Answer:
Br-CH2-CH(CH3)2-C(Cl)H-CH(CH3)2-CHO
Explanation:
The molecule has a total of 14 carbon atoms, 13 hydrogen atoms, and 1 bromine atom. The carbon atoms are arranged in a chain with a methyl group attached to the second carbon atom, a chlorine atom attached to the third carbon atom, and two methyl groups attached to the fourth carbon atom. The fifth carbon atom has a carbonyl group attached to it.
The molecule is an aldehyde, which means that it has a carbonyl group (C=O) at the end of the chain. The carbonyl group is polar, and the oxygen atom has a partial negative charge. The hydrogen atom has a partial positive charge. This polarity makes the aldehyde group susceptible to nucleophilic attack.
The bromine and chlorine atoms are both electrophilic, which means that they have a partial positive charge. This makes them susceptible to nucleophilic attack.
The methyl groups are non-polar and do not have any significant reactivity.
The molecule is a chiral molecule, which means that it has a mirror image that is not superimposable on itself. This is because the carbon atom with the carbonyl group is attached to four different groups.
The molecule is a liquid at room temperature and has a strong odor. It is used in a variety of products, including perfumes, flavorings, and plastics.
When fat comes in contact with sodium hydroxide, it produces soap and glycerin. Determine whether this is a physical change or a chemical change. Explain your
Answers
Answer:
It is a chemical change
Explanation:
The combination of sodium hydroxide and fat yields soap and glycerine.
We have to remember that one of the characteristics of a chemical change is that new substance(es) is/are formed. We have to look out for this when considering any process.
We can see here that new substances were formed (soap and glycerine). Based on this, we can assert that a chemical change has taken place.
A student in CEM143 was doing a fractional distillation when she noticed that the temperature suddenly started to drop. What could have caused the decrease in the temperature
Answers
Explanation:
A process where two or more number of miscible liquids present in different fractions are separated by boiling at different temperatures is called fractional distillation.
The sudden decrease in temperature is because the compounds having lower boiling point have completed the distillation before vapor of the higher boiling point can actually fill the distillation head.
Which is an unavoidable error in this experiment?
Responses
A The tube in the machine is on it's seventh run and may contain the remains of old experiments.The tube in the machine is on it's seventh run and may contain the remains of old experiments.
B Injection of the sample into the machine requires a certain minimum time.Injection of the sample into the machine requires a certain minimum time.
C Darryl washed the sample with the wrong solvent.Darryl washed the sample with the wrong solvent.
D Darryl set the temperature on the machine to 350°C instead of 400°CDarryl set the temperature on the machine to 350°C instead of 400°C
Answers
Match the effects on the concentration if SO2 gas when the following changes occur after initial equilibrium has been established in this system:
2SO2(g) + O2(g) — 2SO3(g) + 46.8 kcal
(increase, decrease, no change)
Pressure increases
Increase [O2]
Increase the volume of chamber
Adding a catalyst
raising the temperature
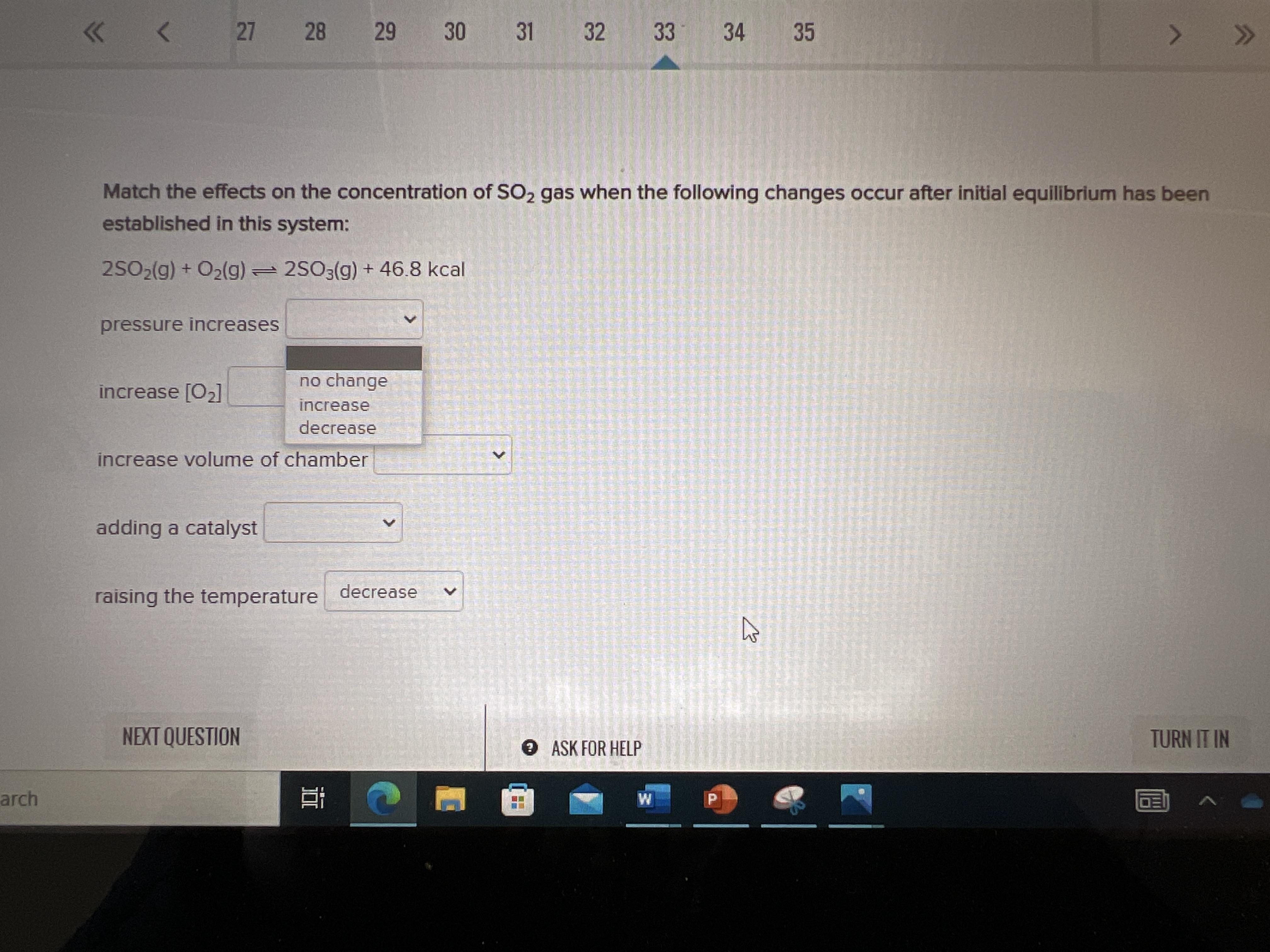
Answers
Answer:
The effects on the concentration of SO3 gas when the following changes occur after initial equilibrium has been established in this system (N.C. = no change) by adding a catalyst.
2SO2(g) + O2(g) 2SO3(g) + 46.8 kcal
So that even though there is an addition of catalyst, no change in reactants or products has occurred because catalyst only provides a faster pathway for the reaction to occur.
Explanation: i think
What atoms have the most positive overall charge to least positive
overall charge.
Atom B: 24 protons, 18 electrons
Atom P: 7 protons, 10 electrons
Atom X: 104 protons, 102 electrons
Atom Q: 15 protons, 16 electrons
Answers
Who used scientific investigations to study atoms?
Check all that apply.
Dalton
Democritus
Rutherford
Thomson

Answers
Answer:
Rutherford used scientific investigation to study atoms.
Scientist who used scientific investigations to study atoms is Rutherford.
What are atoms?Atoms are the basic fundamental or functional unit of any substance present in the nature.
Rutherford is also known as the father of nuclear physics and he did scientific investigation on the massive part of atom called nucleus he discovered that there are two types of radiation, coming from the uranium atom are alpha and beta particles.
Scientific investigation is a method in which scientist will study, perform and observe results for the experiment.
Hence Rutherford used scientific investigation.
To know more about Scientific investigation, visit the below link:
https://brainly.com/question/17216882
What causes a blue block to appear blue in the sunlight?
Group of answer choices
the blue block reflects all blue light and absorbs all other light
the blue block absorbs blue light and reflects all other light
only blue light is refracted and all other light is absorbed into the block
only blue light transmits through the block and all other light is reflected
Answers
Answer:
sadgsddagd
Explanation:
how are you doing?
In a chemical reaction, [_____] are the substances present after the reaction.
Answers
Answer:
Products
Explanation:
Which of the following
best describes the cell
wall of a plant cell?
A. sturdy
B. unstable
C. flimsy
D. weak

Answers
Answer:
A. Sturdy
Explanation:
Unlike every other answer, the cell wall is the part of the cell that acts as a strong barrier of the cell. In this case, it is sturdy.
AIDS is one disease caused by a virus infection. The virus attacks immune system cells known as T cells.
Based on your observations from the Gizmo, how would you explain the data shown on this graph?
Answers
Answer:
yes,its true
Explanation:
I just want to say first off
the immune cell known as T cell also known as white blood cells
Since the first main attack from the bodies response against a virus is deploying T cells to attack
CD4 cells are the basic T cells and HIV virus which is AIDS will start to "Eat itself inside" Ofc not they will use protein spikes to form their way in
they start to create copies of themselves inside the T cell itself and creating a new protein called Env to form their ways in ofc
They are able to bypass CD4 cells is because its a basic cellular receptor for T cells making them really deadly
They now will start to attack other white cells and deploying more and more of their virus cells
Question 21 of 30
What is the frequency of an electromagnetic wave that has a wavelength of
3.7 x 10-11 m in a vacuum? (The speed of light in a vacuum is 3.00 × 108
m/s.)
OA. 8.1 x 1018 Hz
B. 1.2 x 10-19 Hz
OC. 1.1 x 102 Hz
OD. 2.7 x 1010 Hz
SUBMIT
Answers
The frequency of the electromagnetic wave is 8.1 x 10 ¹⁸ Hz.
The speed of light in a vacuum is given as 3.00 x 10⁸ m/s. The speed of light is also related to the wavelength and frequency of the electromagnetic wave by the equation:
c = λν
where c is the speed of light, λ is the wavelength, and ν is the frequency.
Rearranging the equation to solve for frequency, we get:
ν = c/λ
Substituting the values given in the problem, we get:
ν = (3.00 x 10⁸ m/s) / (3.7 x 10⁻¹¹ m)
ν = 8.1 x 10 ¹⁸ Hz
Therefore, the frequency of the electromagnetic wave is 8.1 x 10 ¹⁸ Hz, and the correct answer is (A) 8.1 x 10 ¹⁸ Hz.
To learn more about electromagnetic wave here
https://brainly.com/question/29774932
#SPJ1
Please answer asap. which compound is a sulfate and forms a yellow solution when added to concentrated HCl
Answers
Answer:
When concentrated hydrochloric acid is added to a very dilute solution of copper sulfate, the pale blue solution slowly turns yellow-green on the formation of a copper chloride complex.
Explanation:
Answer: The reversible copper sulfate reaction
When concentrated hydrochloric acid is added to a very dilute solution of copper sulfate, the pale blue solution slowly turns yellow-green on the formation of a copper chloride complex.
Explanation:
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?
Answers
Heat is added to ice at 0 °C. Explain why the temperature of the ice does not change. What does change?When heat is added to ice at 0°C, the temperature of the ice does not change. This happens because all the heat energy is used up in overcoming the intermolecular forces of attraction (hydrogen bonds) that exist between the water molecules in ice.
As a result, the ice undergoes a phase change, from a solid to a liquid. This process is called melting. During melting, the temperature of the ice remains constant at 0°C because all the heat energy is used up in overcoming the intermolecular forces of attraction.The energy required to melt ice is known as the heat of fusion. The heat of fusion is the amount of heat energy required to change 1 kilogram of a solid into a liquid at its melting point. For water, the heat of fusion is 334 kJ/kg. This means that 334 kJ of heat energy is required to melt 1 kg of ice at 0°C. Therefore, during the melting of ice, the temperature of the ice does not change, but the internal energy of the ice does change, and this is manifested in the change of phase from a solid to a liquid.In summary, when heat is added to ice at 0°C, the temperature of the ice does not change, and all the heat energy is used up in overcoming the intermolecular forces of attraction between the water molecules in ice. This results in the melting of ice without any change in temperature.For such more question on molecules
https://brainly.com/question/475709
#SPJ8