Answers
Answer:
Jupiter and Saturn has ammonia clouds in its atmosphere since the molecules of ammonia gas that are present in their atmospheres condense to form clouds. However, these ammonia clouds are more visible in Jupiter than in Saturn because of their lower altitude in Saturn.
Answer:
A
Explanation:
i took the exam
Related Questions
Convert the following to Celsius
32°F
Answers
Answer:
0 degrees celcius love yall
can i get brainliest
What is the identity of a cation solution that burns in a flame test with a mix of red and yellow, but viewed through a cobalt filter the flame is red?
Answers
The identity of a cation solution that produces a mix of red and yellow colors in a flame test, but appears red when viewed through a cobalt filter, can be attributed to the presence of the strontium (Sr) cation.
During a flame test, different metal cations emit characteristic colors due to the excitation of electrons and their subsequent emission of light. Strontium, in particular, is known to produce a vibrant red color in flame tests.
The presence of both red and yellow colors indicates the possibility of multiple metal cations in the solution. While the specific metal responsible for the yellow color is uncertain, it could potentially be sodium or another metal that emits a yellow flame.
When the flame is viewed through a cobalt filter, which absorbs yellow wavelengths of light, the yellow color is filtered out, resulting in only the red color being observed. Since strontium is known for its distinctive red flame color and its emission is not affected by the cobalt filter, it is likely the metal cation responsible for the observed red color. Therefore, based on these characteristics, the identity of the cation solution is most likely strontium (Sr).
Know more about cation solution here:
https://brainly.com/question/30754382
#SPJ8
A bowl of melted butter is allowed to stand until it solidifies at room temperature. Which of the following is true of this process? Answer choices: The butter undergoes a chemical change as it solidifies. The process does not obey the law of conservation of energy. The process has heat flow to the surroundings. The process is endothermic.
Answers
Answer
The process has heat flow to the surroundings.
Explanation
Note that all phase changes are accompanied by changes in the energy of a system. Changes from a more-ordered state to a less-ordered state (such as a liquid to a gas) are endothermic. Changes from a less-ordered state to a more-ordered state (such as a liquid to a solid) are always exothermic.
A melted butter is in liquid phase and is allowed until it changes to solid at room temperature, this change is exothermic and exothermic process is accompanied by releasing heat to the surroundings.
Hence, the only process in the options that is true is:
The process has heat flow to the surroundings.
A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, how many moles of Cu does it contain
Answers
Answer:
4.0561×0.03÷63.5= 0.001916 mol cu
A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, moles of Cu does it contain 0.001916 mole of Cu.
What is mass ?It is the most fundamental characteristic of matter and one of the fundamental quantities in physics.
Mass is a term used to describe how much matter is there in a body. The kilogramme is the SI unit of mass (kg). A body's mass does not alter at any point in time.
Given mass of coin = 4.0561g , moles =? , Cu by mass= 3.00%
4.0561×0.03÷63.5= 0.001916 mole Cu.
Thus, A coin is 3.00% Cu by mass. If the mass of the coin is 4.0561g, moles of Cu does it contain 0.001916 mole of Cu.
To learn more about mass, refer to the below link:
https://brainly.com/question/13592376
# SPJ2
Which one is it I don’t know

Answers
Using the prefix method for covalent compounds, the names of the given compounds are shown below:
LIF: Lithium fluoride
Cl2O7: Dichlorine heptoxide
N2O3: Dinitrogen trioxide
SF6: Sulfur hexafluoride
Na3PO4: Sodium phosphate
What are covalent compounds?A covalent bond is described as a chemical bond that involves the sharing of electrons to form electron pairs between atoms.
The properties of covalent compounds includes:
The boiling/melting points of covalent compounds are low.covalent compounds are soft in nature and relatively flexible.covalent compounds do not possess electrical conductivity.Learn more about covalent compounds at:
https://brainly.com/question/3447218
#SPJ1
_5_ NH3 + _2_ O2 → __ NO + __ H2O
Answers
Answer: should be 4, 5, 4, 6
Explanation:
Calculate the maximum mass of aluminium metal that can be extracted from 25.5 tonnes of aluminium oxide?
Answers
One mole of aluminum oxide or Al₂O₃ is 102 g/mol . It contains 54 g of Al metal. Thus, 25.5 tones of aluminum oxide contains 13.5 tones of Al. Therefore, the maximum mass of aluminum that can be extracted is 13.5 tones.
What is aluminum?Aluminum is 13 the element in periodic table. It is an electropositive element and exhibit metallic properties. Aluminum easily forms its oxides by reacting with atmospheric oxygen.
The molar mass of aluminum oxide Al₂O₃ is 102 g/mol. The atomic mass of Al is 27 g/mol. Thus 102 g of Al₂O₃ contains 54 g of Al. Therefore, the mass of Al in 25.5 tones or 25.5 ×10⁶ g of Al₂O₃ is calculated as follows:
mass of Al = (25.5 ×10⁶ g × 54 g) / 102 g
= 13.5 ×10⁶ g = 13.5 tones.
Therefore, the maximum mass of aluminum that can be extracted is 13.5 tones
To find more on aluminum, refer here:
https://brainly.com/question/12768349
#SPJ1
A student made a sketch of a potential energy diagram to represent an endothermic reaction.
A curve line graph is shown. The y axis of the graph has the title Potential Energy and kJ written in parenthesis. The x axis of the graph has the title Reaction Pathway. The curve begins at a higher level and ends at a slightly lower level. A broken horizontal line is shown from a point labelled X on the y axis to the point where the curve begins. Another broken horizontal line is shown from a point labeled Y on the y axis to the point where the curve ends.
Explain, using complete sentences, why the diagram made by the student is correct or incorrect. Be sure to also explain what the values of X and Y represent.
Answers
Based on the description of the potential energy diagram provided, the diagram made by the student appears to be correct.
The potential energy diagram represents the energy changes that occur during a chemical reaction. In an endothermic reaction, the products have higher potential energy than the reactants, meaning energy is absorbed from the surroundings.
The curve line on the graph indicates the energy changes throughout the reaction pathway. It starts at a higher level, representing the initial potential energy of the reactants. As the reaction progresses, the potential energy decreases, indicating the formation of products with lower potential energy.
The broken horizontal line from point X on the y-axis to the point where the curve begins represents the activation energy (Ea) of the reaction. Activation energy is the energy barrier that must be overcome for the reactants to convert into products.
Point X on the y-axis indicates the potential energy of the reactants at the start of the reaction, and the broken line shows the energy required to initiate the reaction.
The broken horizontal line from point Y on the y-axis to the point where the curve ends represents the potential energy of the products. Point Y represents the potential energy of the products at the end of the reaction.
Overall, the student's diagram correctly represents an endothermic reaction, showing the potential energy changes, the activation energy, and the final potential energy of the products. The curve line starts at a higher level (representing the higher potential energy of the reactants) and ends at a slightly lower level (representing the lower potential energy of the products).
For more such questions on potential energy diagram visit;
https://brainly.com/question/23343697
#SPJ8
CHEMISTRY!! 50 POINTS!
There are 5.5 L of a gas present at -38.0 C. What is the temperature if the volume of the gas has changed to 1.30 L?
Answers
Answer:
The answer to this question is 33.8
Which of the following processes is exothermic? A.cooking an egg B. the chemical reaction in a "cold pack" often used to treat injuries a camp fire C. melting of ice D. None of the above is exothermic
Answers
Answer: none of the above
Explanation: all are endothermic
A 200 N force is applied to an object, which then accelerates at 2 m/s². What is the mass of the object?
Answers
The mass of the object that is acted on by a force of 200 N is 100 kg
What is mass?Mass can be defined as the quantity of matter a body contains.
To calculate the mass of the object, we use the formula below.
Formula:
m = F/a................ Equation 1Where:
m = Mass of the objectF = Force of the objecta = Acceleration due to gravityFrom the question,
F = 200 Na = 2 m/s²Substitute these values into equation 1
m = 200/2m = 100 kgHence, the mass of the object is 100 kg.
Learn more about mass here: https://brainly.com/question/19385703
#SPJ1
what step of the scientific method do we decide whether our hypothesis was correct or not
Answers
Your experiment tests whether your prediction is accurate and thus your hypothesis is supported or not. It is important for your experiment to be a fair test.
A pressure chamber in a lab is regulated to STP, when three gases are introduced (CO₂ = 0.39
atm, and H₂O = 37 kPa) what is the pressure of the third gas?
Answers
Answer:
To regulate a pressure chamber in a lab to STP (Standard Temperature and Pressure), the conditions inside the chamber need to be set to a temperature of 0 degrees Celsius (273.15 K) and a pressure of 1 atmosphere (101.325 kPa or 1013.25 hPa).
Let's assume that the three gases introduced into the pressure chamber are carbon dioxide (CO₂), nitrogen (N₂), and oxygen (O₂), and their initial quantities are as follows:
CO₂: 0.39 moles
N₂: 0.25 moles
O₂: 0.36 moles
To regulate the pressure chamber to STP, we need to calculate the total pressure of the gases inside the chamber and adjust it to 1 atmosphere if needed.
First, we need to calculate the total number of moles of gas in the chamber by summing up the moles of each gas:
Total moles of gas = moles of CO₂ + moles of N₂ + moles of O₂
Total moles of gas = 0.39 moles + 0.25 moles + 0.36 moles
Total moles of gas = 1.0 moles
Next, we can calculate the partial pressure of each gas using Dalton's Law of Partial Pressures, which states that the total pressure of a gas mixture is equal to the sum of the partial pressures of each gas:
Partial pressure of CO₂ = moles of CO₂ / total moles of gas * total pressure
Partial pressure of CO₂ = 0.39 moles / 1.0 moles * 1 atmosphere
Partial pressure of CO₂ = 0.39 atm
Partial pressure of N₂ = moles of N₂ / total moles of gas * total pressure
Partial pressure of N₂ = 0.25 moles / 1.0 moles * 1 atmosphere
Partial pressure of N₂ = 0.25 atm
Partial pressure of O₂ = moles of O₂ / total moles of gas * total pressure
Partial pressure of O₂ = 0.36 moles / 1.0 moles * 1 atmosphere
Partial pressure of O₂ = 0.36 atm
Now, we can check if the total pressure of the gases in the chamber is already at 1 atmosphere or if it needs to be adjusted.
Total pressure of gases = sum of partial pressures of each gas
Total pressure of gases = 0.39 atm + 0.25 atm + 0.36 atm
Total pressure of gases = 1.0 atm
Since the total pressure of the gases in the chamber is already 1 atmosphere, no adjustment is needed. The pressure chamber is already regulated to STP.
Explanation:
Rounding 6.42 g to 2 significant figures
Answers
Answer:
6.4
Explanation:
What contributes to water's properties?
Polar molecule
Shape of the molecule
All of the other options are correct
Oxygen is more electronegative than hydrogen
Answers
A 64.0g sample of methanol requires 1.61kJ of heat to raise its temperature from 22.0 ℃ to 32.0℃. Find thespecific heat capacity of methanol.
Answers
Explanation:
The following formula is used to calculate the specific heat of substances:
c = Q/m. ΔT
Where,
c: specific heat (cal/g.°C or J/Kg.K)
Q: amount of heat (cal or J)
m: mass (g or kg)
ΔT: temperature variation (°C or K)
C: heat capacity (cal/°C or J/K)
In the International System (SI), specific heat is measured in J/Kg.K (Joule per kilogram and per Kelvin). However, it is very common to be measured in cal/g.°C (calorie per gram and per degree Celsius).
1 cal = 4.186 J
First, let's transform Joule into Cal:
1 cal ---- 4.186 J
x cal ---- 1,610 J
x = 384.6 cal
Second:
Find ΔT
ΔT = 32 - 22 = 10
Now let's replace these values in the formula:
c = 384.6/64*10
c = 0.6 cal/g.°C
Answer: c = 0.6 cal/g.°C
2. A restaurant offers a $19.99 three course meal special that lets you choose an appetizer,
an entree, and a dessert. There are 8 appetizers, 12 entrees, and 6 desserts from which
to choose. How many different meals are available?
Answers
There are 576 different ways to choose a meal.
Promotional offers. A promotional offer is a specific proposition to clients that specifies a reward and patron behavioral standards for earning a reward. the recognition and trouble of the praise are captured thru a retail transaction, purchaser order, rebate claim, rebate redemption, or different patron interplay. a discount is a difference between the unique charge and the lower charge it's far being bought at. a proposal is a deal wherein a product is normally bought at a reduction.
Calculation:-
There are 8 appetizers, 12 entrees, and 6 desserts.
Number of choices = 8 × 12 × 6
= 576
Learn more about different meals here:-https://brainly.com/question/8880115
#SPJ1
A 300.0 mL quantity of hydrogen is collected over water at 19.5 C and a total atmospheric pressure of 750. mm Hg. The partial pressure of water at this temperature is 17.0 mm Hg
Answers
The partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg (calculated by subtracting the partial pressure of water, 17.0 mm Hg, from the total atmospheric pressure, 750.0 mm Hg).
When a gas is collected over water, the presence of water vapor affects the total pressure observed. In this case, the total atmospheric pressure is given as 750.0 mm Hg, and the partial pressure of water vapor at 19.5°C is 17.0 mm Hg.
To determine the partial pressure of hydrogen, we need to subtract the partial pressure of water vapor from the total atmospheric pressure. Partial pressure refers to the pressure exerted by an individual gas component in a mixture. In this scenario, the collected gas is primarily hydrogen, with water vapor being the other component.
By subtracting the partial pressure of water vapor (17.0 mm Hg) from the total atmospheric pressure (750.0 mm Hg), we can find the partial pressure of hydrogen:
Partial pressure of hydrogen = Total atmospheric pressure - Partial pressure of water vapor
Partial pressure of hydrogen = 750.0 mm Hg - 17.0 mm Hg
Partial pressure of hydrogen = 733.0 mm Hg
Therefore, the partial pressure of hydrogen in the collected gas sample is 733.0 mm Hg.
Know more about hydrogen here:
https://brainly.com/question/24433860
#SPJ8
pls help me in solve this question in chemistry


Answers
The chemical equation for the decomposition of water is:
\(2 H_2O -- > 2 H_2 + O_2\)
To balance this equation, we need to count the number of atoms of each element on both sides of the equation.
On the left side of the equation, we have:
2 hydrogen atoms (2 H₂O)
2 oxygen atoms (2 H₂O)
On the right side of the equation, we have:
2 hydrogen atoms (2 H₂)
2 oxygen atoms (1 O₂)
We can see that the number of hydrogen atoms is already balanced, but the number of oxygen atoms is not. To balance the equation, we need to add a coefficient in front of O2 so that we have the same number of oxygen atoms on both sides.
The balanced equation is:
\(2 H_2O -- > 2 H_2 + 1 O_2\)
A compound is broken down into simpler compounds during a decomposition reaction. Different techniques, such as heating, exposure to light, or the inclusion of a catalyst, can be used to produce this reaction.
The reactant component splits into two or more products, which may be elements or compounds, during decomposition. A synthesis reaction, in which less complex substances join to create a more complex compound, is the antithesis of this reaction.
learn more about decomposition here
https://brainly.com/question/27746877
#SPJ1
calculate ph of a sloution prepared by dissloving 2.05g of sodium acetate, CHCOONa in 92.0mlof 0.15M acetate acid, CH3COON(ag). assume the volume change upon dissloving the solution acetate is neglizible. Ka of CH3Choon is 1.75x10-5
Answers
The pH of the solution is 4.58.
What is pH?
pH which denotes "potential of hydrogen", is described as a scale used to specify the acidity or basicity of an aqueous solution.
In order to find the pH of the solution, we need to use the weak base-strong acid equation:
Kb = Kw / Ka = [CH3COO-][H+] / [CH3COOH]\
Ka = [H+][CH3COOH] / [CH3COO-]
[H+] = sqrt(Ka * [CH3COOH]) = √t(1.75x10^-5 * 0.15) = 1.36x10^-5 M
We then find the concentration of acetate ions from the sodium acetate.
We have 2.05 g of sodium acetate, which is equivalent to 2.05 / (82.03 + (12 + 16 + 1) * 2) = 0.0163 moles.
[CH3COO-] = 0.0163 M
Kb = Kw / Ka = 1.36x10^-5 / 0.0163 = 8.36x10^-10
In conclusion,
pH = pKb + log([CH3COO-] / ([CH3COOH] + [CH3COO-]) = 4.58
Learn more about pH at: https://brainly.com/question/172153
#SPJ1
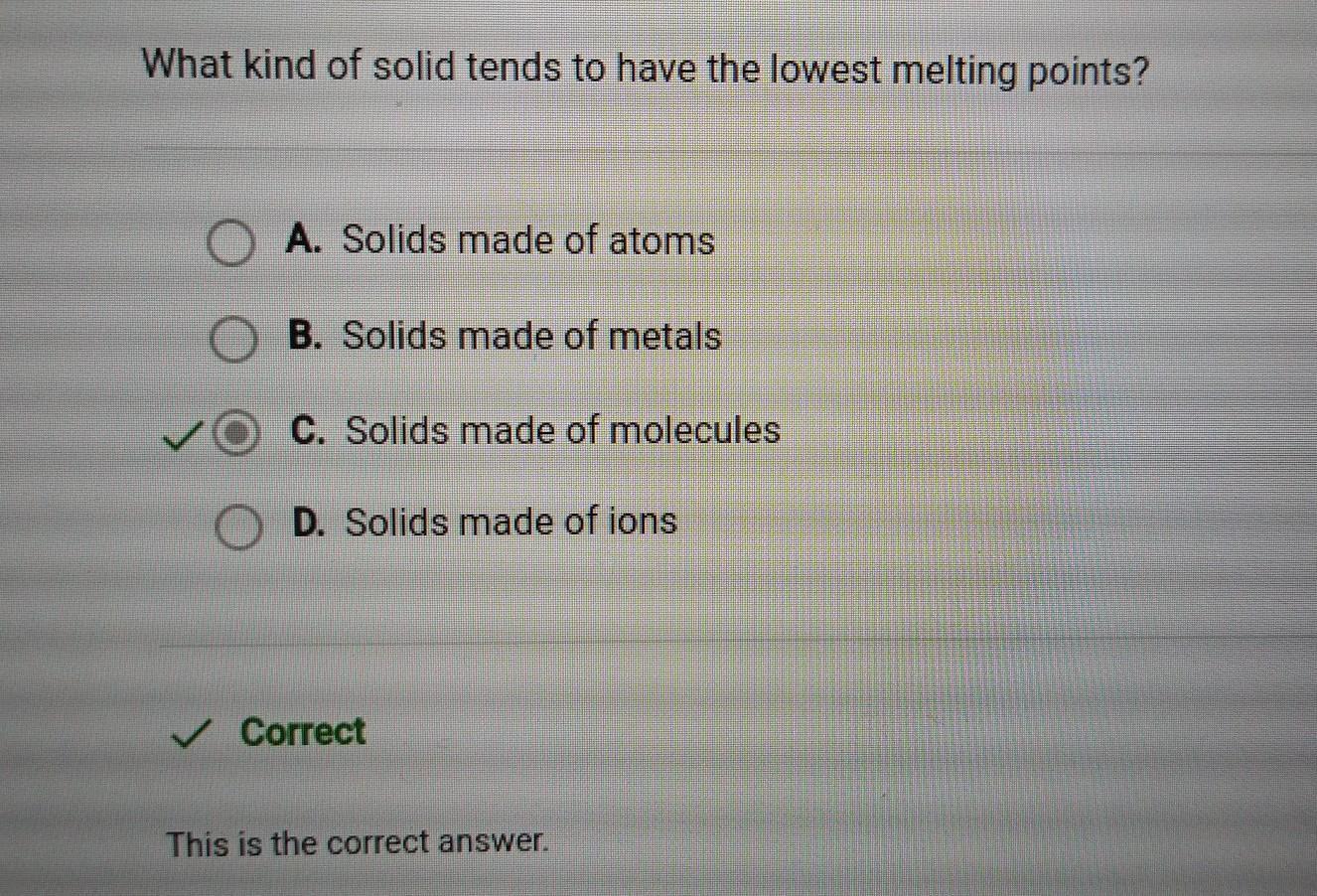
Question 6 of 10
What kind of solid tends to have the lowest melting points?
A. Solids made of ions
B. Solids made of atoms
C. Solids made of molecules
D. Solids made of metals
Answers
Answer:
C. Solids made of molecules
Answer:
C
Explanation:
Solids made of molecules
What is the answer to this question?

Answers
Answer:
DUDE ITS THE THIRD ANSWER FROM THE TOP
....... HOPE THIS HELPS ✌
What is the molar mass of iron (III) oxide?
Answers
Answer:
159.69 g/mol
Explanation:
Here's the answer hope it helps
A Air at a temperature of 134°C and atmospheric pressure flows with a velocity of 7 m/s over
a smooth 5 m long and 1 m wide cast iron plate. Under steady-state conditions, the air-side
surface temperature of the plates is at 120°C. Under the described conditions, determine the
overall heat transfer rate through the plate.
B-If the flat plate in (A) is replaced with a cylinder with the same surface area (heat transfer
area) and the cylinder's length is the same as the width of the plate (1.0 m), what would be the
heat transfer rate for cross air flow over the outside surface of the cylinder?
C. If the flat plate in (A) is replaced with a sphere with the same surface area (heat transfer area),
what would be the heat transfer rate for cross air flow over the outside surface of the sphere?
D- Based on your results, discuss which surface configuration is more effective for heat
Answers
How does electroplating happen?
A. A thin layer of metal is removed from a cathode.
B. Metal ions form a solid layer on a metal cathode.
C. Metal on a cathode forms ions in solution.
D. Metal ions in solution form a precipitate.
(Answer is B.)
Answers
Answer:
B
Explanation:
An electroplating happen when metal ions form a solid layer on a metal cathode. Therefore, option B is correct.
What is an electroplating ?The term electroplating is defined as the process of aligning another metal on the metal. Electroplating is usually used to change the physical properties of an object.
An electroplating is accomplished by an electroplating apparatus that contain a brine solution, a battery, wires, and alligator clips that kept carbon rods attached to the metal to be electroplated and the metal to be layered.
This process is used to give objects increased wear resistance, corrosion protection or aesthetic appeal and greater thickness. While electroplating is like advanced technology. It is primarily used to change the physical properties of an object.
Thus, option B is correct.
To learn more about an electroplating, follow the link;
https://brainly.com/question/20112817
#SPJ5
a stick of butter weighs 0.25lbs. what is the weight of the stick of butter in milligrams?
Answers
A stick of butter weighs 0.25lbs. The weight of the stick of butter in milligrams is 113398.1.
What are milligrams?Milligrams are the measurement quantity that is used to measure a substance in very minute quantities. It is the SI unit of mass. It is equivalent to one thousand grams.
lb is the symbol of a pound. It is used to measure an object in American and British systems of measurement. It is a value of weight.
1 lb = 453592 milligrams
0.25 lbs = 113398.1 milligrams
Therefore, the weight of the stick of butter in milligrams is 113398.1.
To learn more about milligrams, refer to the link:
https://brainly.com/question/8161648
#SPJ1
Assignment Your Unde a professor in a University has Sent you an touration 6 his Inaugural lectore wate a letter to him, showing appreciation for him on halind gesture and Congratulating! his achievements So far
Answers
In this letter, express gratitude to your uncle, a university professor, for his invitation and congratulate him on his achievements.
Here are the steps to be followed:
Express gratitude and appreciation: Begin the letter by expressing your gratitude for your uncle's kind gesture in inviting you to his inaugural lecture. Show genuine appreciation for his thoughtfulness in including you in such an important event.
Congratulate your uncle on his achievements: Extend your heartfelt congratulations to your uncle for his accomplishments thus far. Acknowledge his hard work, dedication, and commitment to becoming a professor at the university. Highlight specific achievements or milestones that you find particularly impressive.
Share your excitement and anticipation: Express your excitement and anticipation about attending his inaugural lecture. Let your uncle know that you are looking forward to being a part of this significant moment in his career and witnessing his expertise in action.
Offer support and encouragement: Offer words of encouragement and support for your uncle's future endeavors. Let him know that you are proud of him and believe in his continued success. Encourage him to keep pursuing his passion and making a positive impact in his field.
Close the letter with warm regards: End the letter with a closing remark and warm regards. You can use phrases such as "Best regards," "With love," or "Sincerely," followed by your name.
Proofread and revise: Before finalizing the letter, review it for any errors or areas that may need improvement. Ensure that the tone is respectful, appreciative, and heartfelt.
Send the letter: Once you are satisfied with the letter, send it to your uncle either via traditional mail or email, depending on your preferred method of communication.
By following these steps, you can write a thoughtful and appreciative letter to your uncle, expressing your gratitude for his invitation and congratulating him on his achievements.
Know more about Letter here:
https://brainly.com/question/18879087
#SPJ8
Please how to do question 4.
Answers
Answer:
all you have to so answer it all done
what properties of a natural resource make it useful for humans as a materials or energy source?
Answers
The properties of a natural resource that make it useful for humans as a material or energy source is the ability to convert mass into energy and vice versa.
What are natural resources?The expression natural resources make reference to all types of matter and energy extracted from nature that can be used to produce goods and services.
Some examples of natural resources include for example irreversible resources such as fossil fuels (i.e., oil, or coal, gas, minerals such as metals, rocks, etc) as well as those based on the use of reversible energy such as eolic air energy, solar radiation or sunlight, soil and hydric resources or water.
Therefore, with this data, we can see that natural resources can be defined as any material and or energy obtained from nature that may be irreversible or reversibly used to produce goods and services.
Learn more about natural resources here:
https://brainly.com/question/24514288
#SPJ1
A golfer putted a golf ball 4.7 ft across a green. How many inches does this represent?
HOW DO WE GET THERE?
How many inches does 4.7 ft represent?
in.

Answers
Answer:
56.4 inches
hope this helps
Explanation: ft to inches conversion