Answers
Raising the temperature of 100 grams of water by 10.0 C is one of those processes which requires more heat energy.
What degree of heat can 100 grams of water withstand?30.514KJ is the amount of heat can 100 grams of water withstand.
Water has a volume of 100 milliliters and weighs 100 grams. (100°C - 27°C) is the temperature difference, which equals 73°C. Water has a specific heat of 4.18 J/g/°C, thus we can apply the calculation below to determine how much energy is required. The necessary energy is equal to 30.514KJ for 100g at 73°C and 4.18 J/g/°C.
What happens to water's thermal energy as it transforms from a liquid to a solid state?Once all of its thermal energy is gone, water freezes, changing from a liquid to a solid. In our daily lives, these kinds of occurrences constantly occur. Puddles, ponds, lakes, and even parts of oceans freeze when the water gets cold enough. In colder climates, the surface water on Earth freezes and solidifies.
To know more about Heat energy visit:
https://brainly.com/question/20038450
#SPJ9
Related Questions
PLEASE HELP WILL MARK BRAINLEST!!!!

Answers
Answer: c????????
Explanation:
The irreversible isomerization A
B was carried out in a batch reactor and the following concentration time data were obtained:
Time vs Concentration data in a Batch reactor
t 0 3 5 8 10 12 15 7.5
mol/h 4 2.89 2.25 1.45 1.0 0.65 0.25 0.07
Determine the reaction order,
, and the specific reaction a rate constant, k, using any method of your choice.

Answers
The reaction order and specific reaction rate constant can be determined by performing the kinetics experiment on irreversible polymerization A. Kinetic experiments can be used to investigate the rate and mechanism of chemical reactions. Chemical kinetics is the study of chemical reactions' speed and pathway.
The term "kinetics" refers to the study of reaction rates, which are determined by measuring the concentration of reactants and products as a function of time.Kinetics experiments can be used to determine the reaction rate and order of reaction. A chemical reaction's rate is defined as the change in the concentration of a reactant or product per unit time. The order of a reaction refers to the number of molecules that must react to produce a product. The order of reaction can be determined by measuring the initial rate of the reaction as a function of concentration.Methods for determining the reaction rate order include the initial rate method, the half-life method, and the integrated rate method. The initial rate method determines the reaction order by measuring the initial rate of the reaction at different reactant concentrations. The half-life method determines the reaction order by measuring the time it takes for the reactant concentration to decrease by half.The integrated rate method determines the reaction order by measuring the concentration of the reactant or product at different times.The specific rate constant can be determined by using the Arrhenius equation, which relates the rate constant to the activation energy, temperature, and frequency factor. The frequency factor can be determined by measuring the rate constant at different temperatures.For such more question on polymerization
https://brainly.com/question/1602388
#SPJ8
A parasitic way of life can be best demonstrated by the feeding adaptations of the spider.
TRUE OR FALSE
Answers
The given statement "A parasitic way of life can be best demonstrated by the feeding adaptations of the spider." is False. Because, While some spiders are parasites, not all spiders are parasitic.
Additionally, many spiders are not even true parasites, as they typically do not harm their host organism. Spiders are typically classified as predators, as they feed on other insects and arthropods. While some spiders may occasionally feed on the blood of larger animals, such as birds or mammals, this behavior is not typically considered parasitic, as it does not involve a long-term relationship between the spider and the host organism.
To know more about parasites, here
brainly.com/question/22589174
#SPJ1
Some atoms have double bonds. Click Remove All, and then add a double bond and two single bonds from the Bonding options.
This molecule now has four bonds, which means the central atom has eight valence electrons. However, these valence electrons are arranged in only three directions around the central atom. Note the bond angles of this molecule.
Remove one of the single-bonded atoms and replace it with a lone pair. How is the remaining bond angle affected by the change?
The bond angle decreases to 109.5°.
The bond angle remains 120°.
The bond angle increases to 180°.
The bond angle remains 109.5°.
Answers
Answer: The bond angle remains 120
Explanation:
Using dimensional analysis, calculate the number of oxygen atoms in 3.25 L of acetic acid (HC2H3O2, density = 1.05 g/mL)?
Answers
The number of oxygen atoms in 3.25 L of acetic acid (HC₂H₃O₂, density = 1.05 g/mL) is 6.84 × 10²⁵.
To calculate the number of oxygen atoms in 3.25 L of acetic acid using dimensional analysis, we need to follow these steps:
1. Convert the volume of acetic acid from liters to milliliters using the conversion factor 1 L = 1000 mL:
3.25 L × (1000 mL / 1 L) = 3250 mL
2. Use the density of acetic acid to convert the volume in milliliters to mass in grams:
3250 mL × (1.05 g / 1 mL) = 3412.5 g
3. Use the molar mass of acetic acid (60.05 g/mol) to convert the mass in grams to moles:
3412.5 g × (1 mol / 60.05 g) = 56.82 mol
4. Use Avogadro's number (6.022 × 10²³ atoms/mol) to convert the moles of acetic acid to molecules of acetic acid:
56.82 mol × (6.022 × 10²³ molecules / 1 mol) = 3.42 × 10²⁵ molecules
5. Finally, use the fact that there are two oxygen atoms in each molecule of acetic acid to find the number of oxygen atoms:
3.42 × 10²⁵ molecules × (2 atoms / 1 molecule) = 6.84 × 10²⁵ atoms
Therefore, there are 6.84 × 10²⁵ oxygen atoms in 3.25 L of acetic acid.
know more about acetic acid here
https://brainly.com/question/15202177#
#SPJ11
An early arrangement of the then known elements was proposed by a British scientist John Newlands, which he called the Law of Octaves. Like other scientists at the time, Newlands arranged the elements in order of increasing atomic mass and noted that every eighth element had similar physical/chemical properties. In the modern Periodic Table, which of the following represents the last pair of elements for which Newlands' Law of Octaves would hold true?
Answers
Lewis dot structure for SO2
Answers
The SO2 Lewis structure would consist of two oxygen (O) atoms and one sulfur atom.
Lewis dot structure for SO2Both the sulfur and oxygen atoms have six reactivity electrons. The molecular geometry of sulfur dioxide is a bent form. The sulfur-to-oxygen ratio in sulfur dioxide. Sulfur dioxide and SiCl4 have 1 lone pair and 2 bond pairs.SO2 molecular addition is considered to V-shaped or bent. otherwise, the electron geometry of sulfur dioxide is in the shape of a trigonal planar. The three pairs of bonding electrons lie at an angle of 119o.
The bond sequence and 1.2 in SO2 and the sulfonyl group, separately. Thus, electrostatic forces (and not multiple covalent bonding) give more to the SÀO bond shortening. In the SO2 lewis structure, there are two double bonds linking the sulfur atoms and oxygen atoms. The sulfur dioxide lewis structure is drawn step by step using VESPR
So we can conclude that The electron dot form of the SO2 molecule is also known as the SO2 Lewis structure.
Learn more about SO2 here: https://brainly.com/question/15654465
#SPJ1
Ca(OH)2 + 2HNO3 → Ca(NO3)2 + 2H2O
Answers
Answer: You have it right
Explanation:
you put the two on the H2O to make it 2H2O and A two on the HNO3 to make it 2HN03 To make it balanced, good job
A metal M react with sulphur ro form MS. If 3.6g of M reacts with 0.09mol of sulphur to form MS . what is the name of M.
Answers
Answer:
Calcium
Explanation:
We must first put down the reaction equation as this will serve as a guide in solving the question.
M(s) + S(s) -----> MS(s)
According to the reaction equation; 1 mole of metal reacts with 1 mole of sulphur. Hence 0.09 moles of sulphur reacts with 0.09 moles of metal.
Now recall that;
Number of moles (n) = mass(m)/ molar mass(M)
Since
mass of metal reacted= 3.6g
Number of moles of metal= 0.09 moles
Then;
Making molar mass of metal the subject of the formula;
Molar mass of metal = mass of metal / number of moles of metal
Molar mass of metal = 3.6g /0.09 moles
Molar mass of metal= 40 gmol-1
The metal having a molar mass of 40gmol-1 is calcium, therefore the metal is calcium.
The pH of a fruit juice is 2.5. Find the hydronium ion concentration, [H₃O⁺]of the juices. Use the formula pH=-log[H₃O⁺]
The hydronium ion concentration [H₃O⁺]is approximately_____ moles per liter
(Use scientific notation. Use the multiplication symbol in the math palates as needed. Round to the nearest tenth as needed.)
![The pH of a fruit juice is 2.5. Find the hydronium ion concentration, [HO]of the juices. Use the formula](https://i5t5.c14.e2-1.dev/h-images-qa/contents/attachments/qb7bOARAdlxF60hhWZ7GCdonMHQqdnNy.jpeg)
Answers
The hydronium ion concentration [H₃O⁺]is approximately 3.16 x 10^-3 moles per liter.
The pH of a solution is a measure of hydrogen ion concentration, which in turn is a measure of its acidity.
The pH of a fruit juice is 2.5.
To calculate the pH of an aqueous solution you need to know the concentration of the hydronium ion in moles per liter (molarity).
By the formula,
pH=-log[H₃O⁺]
2.5 = -log[H₃O⁺]
-2.5 = log[H3O+]
[H3O+] = antilogarithm(-2.5)
[H3O+] = 3.16 x 10^-3 moles.
To learn more about pH of a solution visit:
https://brainly.com/question/28113949
#SPJ4
The hydronium ion concentration [H₃O⁺] of a fruit juice with pH 2.5 is 3.16 × 10⁻³ moles per litre.
The pH of a solution is defined as the hydrogen ion concentration present is a substance. The more the hydrogen ion concentration, more is the acidity of the substance.
In order to calculate the pH of an aqueous solution, firstly we must know the concentration of hydronium ion [H₃O⁺] in moles per litre which is given by the formula
pH = -log [H₃O⁺]
From here, another defination of pH is that pH is equl to the negative logarithm of the hydronium ion concentration.
According to the question,
given
pH of fruit juice = 2.5
To find: hydronium ion concentration [H₃O⁺]
Thus, by formula
2.5= -log[H₃O⁺]
[H₃O⁺]= antilogarithm (-2.5)
[H₃O⁺] = 3.16 × 10⁻³ moles per litre.
Thus, the hydronium ion concentration [H₃O⁺] is 3.16 × 10⁻³ moles per litre.
To know more about hydronium ion concentration here
https://brainly.com/question/28113949
#SPJ4
Carbohydrates are formed by plants converting water and carbon dioxide into glucose and oxygen, in the photocatalyzed process calledQuestion 18 options:A) peptide formation.B) photosynthesis.C) polymerization.D) dehydration.
Answers
Answer:
B; Photosynthesis
Explanation:
Here, we want to get the process that helps produce glucose and oxygen
The process here is called Photosynthesis
It is the process by which green plants use chloropyhll iin them, catalyzed by sunlight to produce energy in form of glucose
Assume that 0.531 g of diborane is combusted in a calorimeter whose heat capacity (Ccalorimeter) is 7.854 kJ/°C at 23.93°C. What is the final temperature of the calorimeter?
Answers
The final temperature of the calorimeter, given that it has initial temperature of 23.93 °C is 19.19 °C
How do I determine the final temeperature?We'll begin by obtaining the heat of combustion of 0.531 g of diborane. This is shown below:
Mass of diborane = 0.531 gMolar mass of diborane = 27.66 g/molMole of diborane = 0.531 / 27.66 = 0.0192 mole1 mole of diborane combust at -1941 KJ
Therefore,
0.0192 mole of diborane will combust at = 0.0192 × -1941 = -37.2672 KJ
Finally, we shall determine the final temperature of the calorimeter. Details below:
Heat of combustion (H) = -37.2672 KJInitial temperature (T₁) = 23.93 °CHeat capacity ofcalorimeter (C) = 7.854 kJ/°CFinal temperature (T₂) = ?H = C(T₂ - T₁)
-37.2672 = 7.854 × (T₂ - 23.93)
Clear bracket
-37.2672 = 7.854T₂ - 187.94622
Collect like terms
-37.2672 + 187.94622 = 7.854T₂
150.67902 = 7.854T₂
Divide both sides by 7.854
T₂ = 150.67902 / 7.854
T₂ = 19.19 °C
Thus, the final temperature is 19.19 °C
Learn more about temperature:
https://brainly.com/question/14281142
#SPJ1
is there any methods to determine number of water crystallization?
Answers
Answer:
In the formula, we can see that for each unit of copper(II) sulfate, there are five molecules of water associated with it. One way to determine the amount of water of crystallization in a hydrated salt is to use volatilization gravimetry
Given the reaction, how many moles of Z will be produced from 4.85 mol A, assuming excess B?
2 A +3B = 4Y + 5 Z
Answers
Answer:
12.125 moles of Z
Explanation:
Hi! Here, since you only know the number of moles for one of the reactants, you will use the coefficients in the reaction to use the mole ratio to find your answer. Since the question states that there are 4.85 mol A and excess B, A is your limiting reactant. This means that once all of the A is used up in the reaction, you will have your maximum amount of Z left behind because there will only be B left and no A to make more Z.
The mole ratio of A:Z is 2:5, found from the coefficients in the chemical equation(2 moles of A for every 5 moles of Z).
So, start with the information that is given to you (4.85 mol A) and then use the mole ratio, like this:
4.85 mol A(\(\frac{5 mol Z}{2 mol A}\))
This way, the mol A will cancel out, leaving behind mol Z (always put the unit you are looking for on top).
Doing this leads to mol Z = 12.125 mol.
I hope this helps! Good luck with your finals!
As per the given 4.85 moles of A will produce 12.125 moles of Z, assuming excess B.
What is chemical reaction?A chemical reaction is the transformation of one or more substances, known as reactants, into one or more different substances, known as products.
Chemical reactions involve the breaking and forming of chemical bonds between atoms and molecules, causing atoms to rearrange and the chemical and physical properties of the substances involved to change.
From the balanced chemical equation, we can see that 2 moles of A react with 3 moles of B to produce 5 moles of Z.
So the mole ratio of A to Z is 2:5.
Therefore, if 2 moles of A react to produce 5 moles of Z, then 4.85 moles of A will produce:
(4.85 mol A) x (5 mol Z / 2 mol A) = 12.125 mol Z
Thus, 4.85 moles of A will produce 12.125 moles of Z, assuming excess B.
For more details regarding chemical reaction, visit:
https://brainly.com/question/29762834
#SPJ2
How many valence electrons do the halogens have?
A. 8
B. 6
C. 7
D. 2
Answers
You look at the very out orbit
convert 200 grams N to moles
Answers
Answer: 14.278880821321199
Explanation:
Answer:
521.12323
Explanation:
Select the correct image
Trees with light brown bark dominate an ecosystem. The ecosystem has populations of insects that thrive on the tree bark. Over time, some
Insect species developed certain physical characteristics that helped them thrive on the tree bark. These physical characteristics also helped the
Insects defend themselves from predators. Which insect species is probably the species with the new physical characteristics?

Answers
Answer:
I think the insect would be the one in the second photo reading from left to right
Explanation:
the other insects:
the first of the first photo is a change of what would be the external surface of the insect, it is not the insect itself.
In the third photo, the insect has short legs with little grip, which indicates that it does not climb large areas on top, and finally, the 4 image is of an insect that lives in desert areas and is poorly coordinated in areas of high heat. and sand.
These are the reasons why I chose photo number two reading from left to right
Answer:
I would say that your answer would be the second one.( the red one)
Explanation:
because the legs it has seem like it would grip onto trees fairly well.
How many liters of carbon dioxide can be produced if 37.8 grams of carbon disulfide react with excess oxygen gas at 28.85 degrees Celsius and 1.02 atmospheres?
CS2(l) + 3O2(g) yields CO2(g) + 2SO2(g)
2.78 liters
5.95 liters
12.1 liters
11.9 liters
Answers
The volume of carbon dioxide produced is approximately (d) 11.9 liters.
To determine the amount of carbon dioxide (C\(O_2\)) produced when 37.8 grams of carbon disulfide (C\(S_2\)) reacts with excess oxygen gas (\(O_2\)), we need to use stoichiometry and the given balanced chemical equation:
C\(S_2\)(l) + 3\(O_2\)(g) → C\(O_2\)(g) + 2S\(O_2\)(g)
First, we calculate the number of moles of C\(S_2\) using its molar mass:
Molar mass of (C\(S_2\)) = 12.01 g/mol (C) + 32.07 g/mol (S) × 2 = 76.14 g/mol
Number of moles of (C\(S_2\)) = mass / molar mass = 37.8 g / 76.14 g/mol ≈ 0.496 mol
From the balanced equation, we can see that the stoichiometric ratio between (C\(S_2\)) and C\(O_2\) is 1:1. Therefore, the number of moles of C\(O_2\) produced will also be 0.496 mol.
Now we can use the ideal gas law to calculate the volume of C\(O_2\) at the given temperature and pressure. The ideal gas law equation is:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant (0.0821 L·atm/mol·K), and T is the temperature in Kelvin.
Converting the temperature from Celsius to Kelvin:
T(K) = 28.85°C + 273.15 = 302 K
Using the ideal gas law:
V = nRT / P = (0.496 mol) × (0.0821 L·atm/mol·K) × (302 K) / (1.02 atm) ≈ 11.9 L
The correct answer is 11.9 liters.
for more questions on carbon dioxide
https://brainly.com/question/26150306
#SPJ8
how do i solve this question? can i please get any help? id really appreciate it!:)
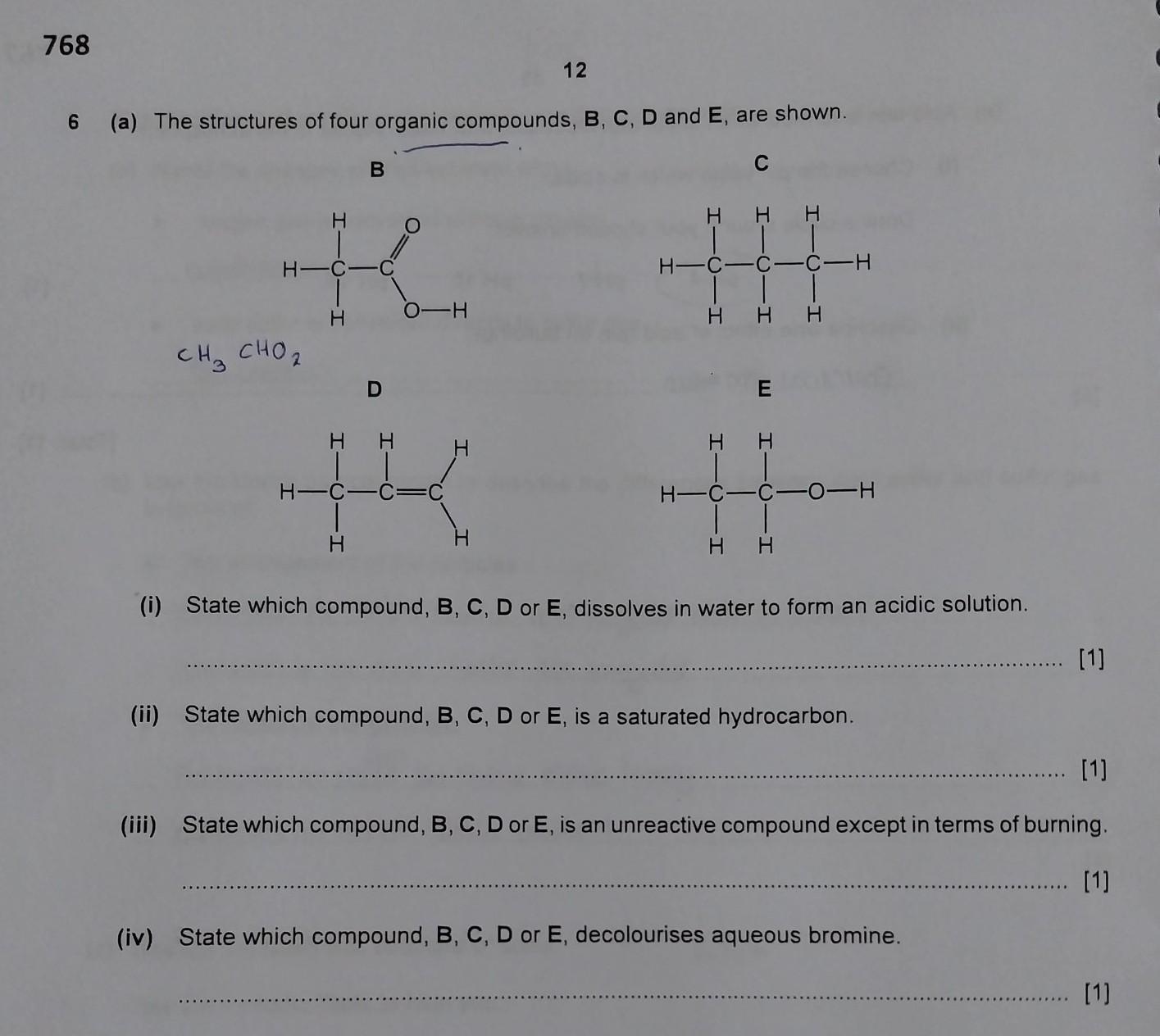
Answers
Answer:
(I) - B
(II) - C
(III) - C
(IV) - D
Explanation:
(I) both of carboxylic acids (B) and alcohols (E) are miscible in H2O, but carboxylic acids increase the acidity of water/solution
(II) Saturated hydrocarbons are from alkane hydrocarbons and they don't have pi bonds, only sigma bonds, and (C) is an alkane (Propane).
(III) Alkanes (C) are unreactive because of sigma bonds which are strong bonds that needs high energy to break
(IV) any unsaturated hydrocarbon (having pi bonds or double bonds, "==" in Lewis Structure) decolourizes Br2 since Br atoms react with the unsaturated hydrocarbon, producing bromo-alkane, for instance, 1-propene (D) + Br2 ---> 1-bromo propane
If the volume is 10 and the mass of water is 9.99 what is the density
Answers
Answer:
0.999
Explanation: divide by 10
Where is the Oort cloud located?
Answers
Answer:
lies far beyond pluto and the most distant edges of kuiper belt
Answer:
in the outermost region of the solar system, beyond Neptune
Explanation:
What is the final temperature after 840 Joules is absorbed by 10.0g of water at 25.0
C?
Answers
The final temperature of the water is: T_final = 45.0°C
We can use the formula for the specific heat capacity of the water to solve this problem:
q = mcΔT
First, we can calculate the initial energy of the water:
q = mcΔT
q = (10.0 g) (4.184 J/g°C) (25.0°C)
q = 1,046 J
Next, we can calculate the final temperature after absorbing 840 J:
q = mcΔT
840 J = (10.0 g) (4.184 J/g°C) (ΔT)
ΔT = 20.0°C
Therefore, the final temperature of the water is:
T_final = T_initial + ΔT
T_final = 25.0°C + 20.0°C
T_final = 45.0°C
To know more about final temperature, here
brainly.com/question/11244611
#SPJ1
17.Which of the following cannot be used to show diffusion?Select one:a. A drop of food coloring added to water.b. Moths attracted to a flame.c. Students leaving school at the end of the day.d. The spreading of the gases from a car's exhaust.
Answers
The definition of diffusion is the process resulting from random motion of molecules from a region of high concentration to a region of low concentration, and the option that does not show this situation of motion is letter B, where we have moths attracted to a flame, there is no high concentration before, is the opposite situation, therefore letter B
Find how many seconds are required to deposit 0.299g of lead metal from solution containing Pb2+ ions if current is 0.627A

Answers
The balanced half-reaction equation for the reduction of Pb2+ ions to lead metal is:
Pb2+ + 2e- → Pb
According to this equation, 2 electrons are required to reduce 1 Pb2+ ion to lead metal.
The molar mass of lead (Pb) is 207.2 g/mol, so the mass of 1 mole of lead is 207.2 g. Therefore, the mass of 0.299 g of lead is:
0.299 g / (207.2 g/mol) = 0.00144 mol
To deposit this amount of lead, we need to transfer 0.00144 mol of Pb2+ ions to lead metal. This requires the transfer of 2 x 0.00144 = 0.00288 moles of electrons.
The amount of electric charge required to transfer 0.00288 moles of electrons is:
Q = n x F = 0.00288 mol x 2 x 96,485.3329 C/mol = 557.4 C
where n is the number of moles of electrons transferred and F is the Faraday constant.
The amount of time required to deposit this amount of lead at a current of 0.627 A is given by the equation:
Q = I x t
where Q is the electric charge in coulombs, I is the current in amperes, and t is the time in seconds. Solving for t, we get:
t = Q / I = 557.4 C / 0.627 A = 888.9 seconds
Therefore, it would take approximately 888.9 seconds (or about 14.8 minutes) to deposit 0.299 g of lead metal from a solution containing Pb2+ ions at a current of 0.627 A.
Plz answer fast Name the four parts of a circuit.
Answers
Answer:
A switch
Battery or cell
Resistor
Rheostat
Appliance or load such as bulb
connecting wires.
Jockey
Inductor
Capacitor
Meter bridge
Potentiometer
Voltimeter
Ammeter
Galvanometer
How could the age be interpreted in a rock in which the blocking temperature has been reached?
Answers
a. List six renewable resources that humans depend on. (0.5 point)
Answers
Answer:
-trees
-solar energy
-hydroelectricity
-wind energy
sorry I can only think or 4 atm
What are ALL full chemical reactions that ensue for each of the following disinfectants (may be more than one for each): (5 points) Cl2 (g) at pH 9
Answers
Answer:
The reactions that might ensue are
Cl\(_{2}\)(g) + H\(_{2}\)O ---------- > HOCl + HCl
HOCl -------- > \(H^+\)(aq) + O\(CI^-\)(aq)
Cl2(g) + \(OH^-\)(aq) ----- > O\(CI^-\)(aq) + HCl
Explanation:
The Disinfectant Given is
CI2 (g) at PH 9
Determine the chemical reactions that might ensue
due to the High concentration of \(H^{+} (aq)\) the formation of \(OCI^-\)(aq) will be better and this will decrease the power of the disinfectant.
Hence the chemical reactions that might ensue can be represented below as :
Cl\(_{2}\)(g) + H\(_{2}\)O ---------- > HOCl + HCl
HOCl -------- > \(H^+\)(aq) + O\(CI^-\)(aq)
Cl2(g) + \(OH^-\)(aq) ----- > O\(CI^-\)(aq) + HCl
A student requires 2.00 L of 0.100 M NH4NO3 from a 1.75 M NH4NO3 stock solution. What is the correct way to get the solution?
Use M subscript i V subscript 1 equals M subscript f V subscript f..
Measure 114 mL of the 1.75 M solution, and dilute it to 1.00 L.
Measure 114 mL of the 1.75 M solution, and dilute it to 2.00 L.
Measure 8.75 mL of the 1.75 M solution, and dilute it to 2.00 L.
Measure 8.75 mL of the 0.100 M solution, and dilute it to 2.00 L.
Answers
Answer:
I believe its B
Explanation:
Answer:
B. Measure 114 mL of the 1.75 M solution, and dilute it to 2.00 L.
Assume that you measured exactly 2.0 mL of the sodium chloride solution into a small test tube. How many moles of
sodium chloride are in the tube?