Answers
Answer:
helium
Explanation:
it has 8 valence electrons which fills its outer energy level making it have a stable arrangement of electrons hence least reactive
Related Questions
A nitrogen-containing compound shows no absorption band at ∼3400cm−1 and no absorption bands between ∼1700cm−1 and ∼1600cm−1. what class of compound is it
Answers
Explanation:
A nitrogen-containing compound that shows no absorption band at around 3400 cm^−1 and no absorption bands between approximately 1700 cm^−1 and 1600 cm^−1 is likely an amide compound.
Amides typically exhibit a characteristic absorption band in the region of 3200-3500 cm^−1 due to the N-H stretching vibration. The absence of this absorption band suggests the absence of N-H bonds, which rules out compounds like primary or secondary amines.
The absence of absorption bands between 1700 cm^−1 and 1600 cm^−1 eliminates functional groups such as carbonyl compounds (e.g., aldehydes, ketones, carboxylic acids, esters) and imines, which typically exhibit absorption in this region.
Therefore, based on the given information, it can be inferred that the compound is likely not an amine, carbonyl compound, or imine. Other classes of compounds that do not possess these characteristic absorption bands would need to be considered.
Which of the following molecules is the least polar?
A) H-H
B) H-CI
C) H-F
D) H-I
Answers
Answer: A) H-H is least polar
Explanation:
Electronegativity is defined as the property of an element to attract a shared pair of electron towards itself. The more is the difference in electronegativity, the more polat the bond is.
1. H-H : Electronegativity difference = electronegativity of hydrogen - electronegativity of hydrogen =2.1 -2.1= 0
2. H-Cl : Electronegativity difference = electronegativity of chlorine - electronegativity of hydrogen = 3-2.1 = 0.9
3. H-F : Electronegativity difference = electronegativity of fluorine - electronegativity of hydrogen= 4-2.1= 1.9
4. H-I : Electronegativity difference = electronegativity of iodine - electronegativity of hydrogen= 2.5 -2.1= 0.4
Thus the least electronegativity difference between the bonded atoms is in H-H , this is least polar.
For the following reaction, 22.3 grams of sulfur dioxide are allowed to react with 5.42 grams of water.
sulfur dioxide (g) + water (l)→ sulfurous acid (H₂SO3) (g)
What is the maximum amount of sulfurous acid (H₂SO3) that can be formed? 24.7 grams
What is the FORMULA for the limiting reagent? H₂O
What amount of the excess reagent remains after the reaction is complete? 3.01 grams
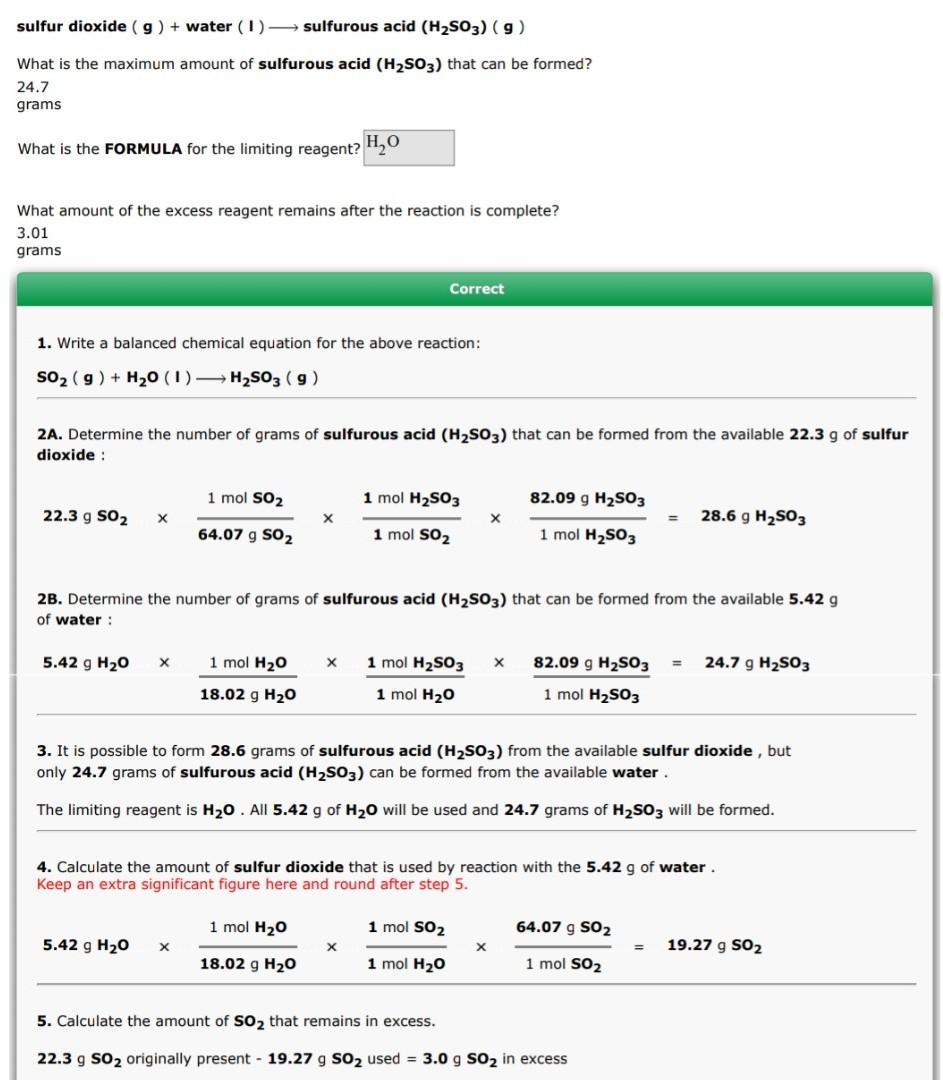
Answers
Explanation:
# grams Sulfuric Acid formed from 22.3 g Sulfur Dioxide
\(23.2 \: g \: SO₂ \times \frac{1 \: mol \: SO₂)}{64.07g \: SO₂} \times \frac{1 \: mol \: H₂SO₃}{1 \: mol \: SO₂)} \times \frac{82.09 \: g \: H₂SO₃}{1 \: mol \:SO₂ } = 28.6 \: g \: H₂SO₃\)
# grams Sulfuric Acid formed from 5.42 g Water
\(5.42 \: g \: SO₂ \frac{1 \: mol \: H₂O)}{18.02g \: H₂O} \times \frac{1 \: mol \: H₂SO₃}{1 \: mol \: H₂O)} \times \frac{82.09 \: g \: H₂SO₃}{1 \: mol \:H₂SO₃ } = 24.7 \: g \: H₂SO₃\)
# Sulfur Dioxide used by reaction 5.42 g Water
\(5.42 \: g \: H₂O \times \frac{1 \: mol \: H₂O)}{18.02g \: H₂O} \times \frac{1 \: mol \: SO₂}{1 \: mol \: H₂O)} \times \frac{64.07 \: g \: SO₂}{1 \: mol \:SO₂ } = 19.27 \: g \: SO₂\)
# of SO₂ in Excess
\( 23.2 \: g \: SO₂ - 19.27 \: g \: SO₂ = 3.0 \: g \: SO₂\)
Iron (Fe) is a/n:
Element
Mixture
Compound
Solution
Answers
Answer:
Element
Explanation:
Iron is an element
Without checking the detailed numbers, please arrange the ionic compounds CsI, MgO, NaCl, and AlN in order of increasing lattice energy.
Answers
For the above compounds, the order of increasing lattice energy is MgO, NaCl, CsI, and AlN.
Which is ionic? MgO or NaCl?Because the ionic species in MgO have greater charge (Mg+2 and O2- as opposed to Na+ and Cl-), the ionic bond is stronger than it is in NaCl. As a result, MgO has greater ionic connections than NaCl. MgO is hence more ionic than NaCl.
whose MgO lattice energy is the highest?The size of the ions involved has an inverse relationship with lattice energy in ionic compounds. MgO has the highest lattice energy because Mg2+, the smallest of the four ions (because anion is the same in all), is present.
To know more about lattice energy visit:-
https://brainly.com/question/18222315
#SPJ1
How much heat is released when 60.0 G of steam at 235°C is converted to water at 100°C?
Answers
Answer:12668 J.
Explanation:
First of all, steam is there so in order to convert it into water it must have to release latent heat of vaporization which is equal to approximately 2450 kJ/kg or 2450 J/g.
So, the calculation is given below.
Heat released during conversion of steam at 100°C to water at 100°C= m*L
= 5*2450= 12250 J.
Heat released during conversion of water at 100°C to water at 80°C= m*Cp*(∆T)
= 5*4.18*20= 418 J.
So total heat released= 12668 J.
hope this helps:)!!!
The heat energy released or absorbed by a system can be calculated using calorimetric equation. The heat is released when 60.0 g of steam at 235°C is converted to water at 100°C is 33858 J or 338 KJ.
What is calorimetry?Calorimetry is an analytical tool used to determine the heat energy q absorbed or released in a reaction system. The calorimetric equation connecting mass of the reactant m, specific heat capacity c and temperature difference ΔT is related by the expression:
q = mcΔT.
The mass of steam is given 60 g and temperature difference is from 235 to 100 °C thus 135 °C and the specific heat capacity of water or steam is 4.18 J/°C. The heat energy released is calculated as follows:
q = 60 g × 4.18 J/°C × 135 °C
= 33858 J
Hence, heat is released when 60.0 g of steam at 235°C is converted to water at 100°C is 33585 J.
To refer more on calorimetry, refer the link:
https://brainly.com/question/11477213
#SPJ2
What is the formula for iron (II) sulfate hexahydrate?
Answers
The formula for iron (II) sulfate hexahydrate
\(is\)
FeH12O10S

What volume of water is required to prepare 0.1 M H3PO4 from 100 ml of 0.5 M solution?
Answers
take mol divide by 0.1 to find volume
Brainliest will be rewarded!

Answers
Option B, where [OH-] is 1.0 x 10-13 mol dm-³3, is the only one that can be considered basic. Therefore, Option B is the correct answer.
To determine whether a solution is basic or acidic at 25 °C, we can compare the concentration of hydroxide ions ([OH-]) with the concentration of hydronium ions ([\(H_3O\)+]). In a neutral solution, the concentrations of [\(H_3O\)+] and [OH-] are equal, resulting in a pH of 7.
Option A states that the concentration of [\(H_3O\)+] is 1.0 x 10-3 mol dm-3. Since [\(H_3O\)+] represents the concentration of hydronium ions, this solution would be acidic because the concentration of [\(H_3O\)+] is higher than [OH-], indicating an excess of hydronium ions.
Option B states that the concentration of [OH-] is 1.0 x 10-13 mol dm-³3. In this case, [OH-] is higher than [\(H_3O\)+], indicating an excess of hydroxide ions. Therefore, this solution would be considered basic.
Option C states that the solution has a pH of 4.00. A pH of 4.00 is below the neutral pH of 7, indicating an excess of hydronium ions and an acidic solution. Therefore, this option does not represent a basic solution.
Option D states that the concentration of [\(H_3O\)+] is 1.0 x 10-13 mol dm-3. Similar to Option A, this concentration of [\(H_3O\)+] indicates an acidic solution, not a basic one.
Option B
For more such question on basic visit:
https://brainly.com/question/29886197
#SPJ8
Answer:
D is the correct answer
Explanation:
In order for a solution to be basic at 25 C, the H+ concentration has to be less than the OH- concentration, and given that H+ times OH- is 10^-14, we deduce that H+ must be less than 10^-7 for the solution to be acidic. Thus, A can be eliminated, and so can C. With B, we calculate an H+ concentration of 0.1M, which also fails to be less than 10^-7
Thus, D is the correct answer and we can verify that as H+ is less than 10^-7.
Note: I do not know why my previous answer was deleted for "being incorrect", and i'm not sure how the incorrect answer was "expert verified", but I am as certain that D is the correct answer as i am sure of 3*(4+5-1) being equal to 24.
An ion charge of -1 in the 4th period
What element is this?
Answers
Every day Sam goes to Dunkin' Donuts and orders a medium iced Coffee with four sugars OR he orders a hot
coffee with four sugars.
He notices that the iced coffee is never as sweet as the hot coffee. Why is this? Use complete sentences and
unit vocabulary to explain.
Answers
In the iced coffee waters it down:)
Starting with 0.3500 mol CO(g) and 0.05500 mol COCl2(g) in a 3.050 L flask at 668 K, how many moles of CI2(g) will be present at equilibrium?
CO(g) + Cl2(g)》COCl2(g)
Kc= 1.2 x 10^3 at 668 K
Answers
At equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
1: Write the balanced chemical equation:
\(C_O\)(g) + \(Cl_2\)(g) ⟶ \(C_OCl_2\)(g)
2: Set up an ICE table to track the changes in moles of the substances involved in the reaction.
Initial:
\(C_O\)(g) = 0.3500 mol
\(Cl_2\)(g) = 0.05500 mol
\(C_OCl_2\)(g) = 0 mol
Change:
\(C_O\)(g) = -x
\(Cl_2\)(g) = -x
\(C_OCl_2\)(g) = +x
Equilibrium:
\(C_O\)(g) = 0.3500 - x mol
\(Cl_2\)(g) = 0.05500 - x mol
\(C_OCl_2\)(g) = x mol
3: Write the expression for the equilibrium constant (Kc) using the concentrations of the species involved:
Kc = [\(C_OCl_2\)(g)] / [\(C_O\)(g)] * [\(Cl_2\)(g)]
4: Substitute the given equilibrium constant (Kc) value into the expression:
1.2 x \(10^3\) = x / (0.3500 - x) * (0.05500 - x)
5: Solve the equation for x. Rearrange the equation to obtain a quadratic equation:
1.2 x \(10^3\) * (0.3500 - x) * (0.05500 - x) = x
6: Simplify and solve the quadratic equation. This can be done by multiplying out the terms, rearranging the equation to standard quadratic form, and then using the quadratic formula.
7: After solving the quadratic equation, you will find two possible values for x. However, since the number of moles cannot be negative, we discard the negative solution.
8: The positive value of x represents the number of moles of \(Cl_2\)(g) at equilibrium. Substitute the value of x into the expression for \(Cl_2\)(g):
\(Cl_2\)(g) = 0.05500 - x
9: Calculate the value of \(Cl_2\)(g) at equilibrium:
\(Cl_2\)(g) = 0.05500 - x
\(Cl_2\)(g) = 0.05500 - (positive value of x)
10: Calculate the final value of \(Cl_2\) (g) at equilibrium to get the answer.
Therefore, at equilibrium, the number of moles of \(Cl_2\) (g) will be 0.2025 mol.
For more such questions on equilibrium, click on:
https://brainly.com/question/517289
#SPJ8
1. Which statement is always true when nuclear fusion occurs?
A. The number of protons in the resulting nucleus is less than in each starting nucleus.
B. The number of protons in the resulting nucleus is double that of a starting nucleus.
C. The combined number of portions and neutrons remains constant.
D. The total number of protons and neutrons in each nucleus remains constant.
•
2. Use the equation to answer the question.
2H + 2H—> 4H + energy
1 1 2
Which statement best describes the mass numbers of the atoms in the reaction?
A. There are two atoms with mass numbers of 2.
B. There is one atom with
a mass number of 2.
C. There are two atoms with mass numbers of 1.
D. There is one atom with a mass number of 1.
•
3. How is the mass number calculated for an atom involved in nuclear fusion?
A. It is the number of electrons.
B. It is the number of protons plus electrons.
C. It is the number of neutrons.
D. It is the number of protons plus neutrons.
•
4. Which change will always take place in nuclear fusion?
A. The total charge will be less than before the fusion took place.
B. The nucleus with a smaller mass than any of the reactants will be produced.
C. A nucleus with a greater mass than any of the reactants will be produced.
D. The total charge will be greater than before the fusion took place.
•
5. Which statement best summarizes the way the sun produces energy?
A. Combustion reactions in the sun release large amounts of chemical energy.
B. Fusion reactions in the sun release large amounts of chemical energy.
C. Combustion reactions in the sun convert small amounts of matter into large amounts of energy.
D. Fusion reactions in the sun convert small amounts of matter into large amounts of energy.
Answers
Answer:
1. The combined number of protons and neutrons remains constant.
2. There are two atoms with mass numbers of 2.
3. It is the number of protons plus neutrons.
4. A nucleus with a greater mass than any of the reactants will be produced.
5. Fusion reactions in the sun convert small amounts of matter into large amounts of energy.
Lewis structure AgBr2
Answers
Answer:
Br-Ag-Br
Explanation:

What mass in milligrams of potassium nitrate is present in 0.250 kg of a 500 ppm aqueous solution of potassium nitrate, KN03(aq)?
Answers
125mg of potassium nitrate is present in 0.250 kg of a 500 ppm aqueous solution of potassium nitrate, KN03(aq).
What is the solution ?A solution is a uniform mixture of one or more dissolved solutes in a solvent. A solvent is a substance that dissolves a solute to produce a homogeneous mixture. The substance that dissolves in a solvent to form a homogeneous mixture is referred to as a solute.
A solution is formed when two chemicals, known as a solvent and a solute, are combined. A solution is formed when a solute and a solvent combine.
1. ppm = mass solute (mg) / mass solution (kg)
2. Re-arrange this equation to find the mass of solute:
mass solute (mg) = ppm x mass solution (kg)
3. Substitute in the values in equation as follows:
mass KNO3 = 500ppm x 0.25kg
= 125mg
Thus, 125mg of potassium nitrate is present in 0.250 kg of a 500 ppm aqueous solution of potassium nitrate, KN03(aq).
To learn more about the solution, follow the link;
https://brainly.com/question/7932885
#SPJ1
what does (I) means in a chemical equation
Answers
Answer:
the l sign means the substance in the chemical equation is a liquid. (hope this helped : D )
How many moles of iron are in 2.34 x 10^22 iron atoms?
Answers
answer
.03886 grams or 3.886 x 10^‐2 grams
steps
avogadros number
6.022×10²³ atoms of ANY ELEMENT = 1 mole
whats different for every element is the number of GRAMS or MASS
1 mole of iron is 55.847 grams
2.34 x 10²² ÷ 6.022×10²³ =
.03886 grams or
3.886 x 10^‐2 grams
Write a balanced equation for the double-replacement precipitation reaction described, using the smallest possible integer coefficients. A precipitate forms when aqueous solutions of cobalt(II) chloride and potassium hydroxide are combined. Use the pull-down boxes to include states such as (8) or (aq).
Answers
The reaction between cobalt (II) chloride and potassium hydroxide can be depicted as follows:
CoCl2(aq)+2KOH(aq)----> Ca(OH)2(s)+2KCl(aq)
A double displacement reaction is one that involves the exchange of ions and leads to the formation of new products. The products that are soluble in water are represented by the symbol (aq) and the ones that are insoluble in the water remain in the (s) or precipitate form along with the chemical formulas.
The double displacement reaction can be depicted as follows:
XY+AB--->AB+AY
The reaction between cobalt (II) chloride and potassium hydroxide can be depicted as follows:
CoCl2(aq)+2KOH(aq)----> Ca(OH)2(s)+2KCl(aq)
To learn more about double displacement reaction check the link below:
https://brainly.com/question/26413416
#SPJ4
Calculate the number of nitrogen atoms in a container of volume 0.50 dm³ at 0 °C. The pressure of the gas is 101 Pa.
Answers
The number of nitrogen atoms in a container of volume 0.50 dm³ at 0 °C. The pressure of the gas is 101 Pa. is approximately 1.47 x 10^19 nitrogen atoms in the given container.
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of gas, R is the ideal gas constant, and T is the temperature in Kelvin.
0.50 dm³ = 0.50 x \(10^-^3\) m³
0 °C = 273 K
n = PV/RT
n = (101 Pa)(0.50 x \(10^-^3\) m³) / [(8.31 J/mol K)(273 K)]
n = 0.0000244 mol
n = (0.0000244 mol)(6.02 x \(10^2^3\)atoms/mol)
n = 1.47 x \(10^1^9\) nitrogen atoms
Learn more about the pressure, gas here.
https://brainly.com/question/31463642
#SPJ1
b. How many kJ of heat are needed to completely vaporize 50.0g of water at 100°C? [Ans:113. kJ]
Answers
The amount, in kJ, of heat needed to completely vaporize 50.0g of water at 100°C is 118.8 kJ.
Heat of vaporization of waterThe heat needed to completely vaporize 50.0g of water at 100°C can be calculated using the following formula:
q = m x Hv
where:
q is the heat needed in joules (J)m is the mass of water in grams (g)Hv is the heat of vaporization of water which is approximately 40.65 kJ/mol at standard temperature and pressure.First, we need to convert 50.0g to moles by dividing by the molar mass of water which is approximately 18.015 g/mol3:
moles of water = 50.0 g / 18.015 g/mol moles of water = 2.776 mol
Thus:
q = (2.776 mol) x (40.65 kJ/mol) q = 112.8 kJ
In other words, 112.8 kJ of heat is needed to completely vaporize 50.0g of water at 100°C.
More on heat of vaporization can be found here: https://brainly.com/question/12625048
#SPJ1
5. The density of water at 4.00°C is 0.967 g/mL. How many molecules of water are present in a 499.8 mL bottle of water? Express your answer to the correct number of significant figures
Answers
There are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
To determine the number of water molecules in the given volume of water, we need to use the relationship between mass, volume, and molar mass of water.
First, we need to find the mass of water in the bottle:
Mass = Density * Volume
Mass = 0.967 g/mL * 499.8 mL = 483.9 g
Next, we need to convert the mass of water to moles using the molar mass of water. The molar mass of water (H2O) is approximately 18.015 g/mol.
Moles = Mass / Molar mass
Moles = 483.9 g / 18.015 g/mol = 26.88 mol
Finally, we can calculate the number of water molecules using Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules = Moles * Avogadro's number
Number of molecules = 26.88 mol * (6.022 x 10^23 molecules/mol) = 1.62 x 10^25 molecules
Therefore, there are approximately 1.62 x 10^25 water molecules in the 499.8 mL bottle of water.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ8
Drag the tiles to the correct locations on the equation. Not all tiles will be used.
Two atoms interact with each other and change as shown by the equation. Complete the equation by filling in the missing parts.
5
2
4
3
1
H+H -
H
He
Li
+
Answers
The equation in the question is: H+H → H + H Complete the equation by filling in the missing parts. missing part is 1 → H+H-2 → →3 → He.
The atomic number of hydrogen is 1, which means it has only one proton in the nucleus and one electron in its shell. Two hydrogen atoms react with each other to form helium. Helium has 2 protons and 2 neutrons in its nucleus and two electrons in its shell. Therefore, the equation is:
H + H → HeIt can be seen that:1. H + H (Reactants)
2. → (Yields or Reacts to form)
3. He (Product)Therefore, the tiles will be arranged as shown below: 1 → H+H-2 → →3 → He
For more question atomic number
https://brainly.com/question/16858932
#SPJ8
Classify the following amine as 1°, 2°, 3° or 4°(primary, secondary, tertiary, quaternary).CH3NH2A. primaryB. secondaryC. tertiaryD. quarternary

Answers
Explanation:
The degree of amines is determined by the number of carbons attached to the nitrogen.
According to the given picture, the answer is A.
Answer:
The answer is A.
write the dissociation equation for lithium hydroxide (lioh, a strong base). use --> for a one way arrow or <--> for a two way arrow.
Answers
The dissociation equation for lithium hydroxide (LiOH), a strong base, is: LiOH (s) → Li+ (aq) + OH- (aq) A dissociation equation is an equation that represents the dissociation of a compound into its ions when dissolved in a solvent.
In this case, LiOH dissociates in water to form Li+ and OH- ions. LiOH is a strong base because it dissociates almost completely in water, producing a high concentration of OH- ions. The dissociation of a strong base is represented by a one-way arrow in the equation, indicating that it proceeds almost completely in one direction.A two-way arrow, <--> , is used in equilibrium reactions, where the forward and reverse reactions occur at the same rate. This is not the case for the dissociation of strong bases like LiOH, where the reaction proceeds almost completely in one direction.
For more such questions on dissociation
https://brainly.com/question/29012577
#SPJ11
A chemist titrates 150.0 mL of a 0.8748M benzoic acid (HC,H,CO₂) solution with 0.3544M KOH solution at 25 °C. Calculate the pH at equivalence. The pK,
of benzoic acid is 4.20.
Round your answer to 2 decimal places.
Answers
HC7H5O2(aq) + KOH(aq) → KC7H5O2(aq) + H2O(l)
The number of moles of benzoic acid used in the titration is:
n(HC7H5O2) = M × V = 0.8748 mol/L × 150.0 mL / 1000 mL/L = 0.13122 mol
The volume of KOH solution required to reach equivalence point can be determined using the stoichiometry of the balanced equation. The stoichiometric ratio of benzoic acid to potassium hydroxide is 1:1. Therefore, the number of moles of KOH required to neutralize all of the benzoic acid is equal to n(HC7H5O2):
n(KOH) = 0.13122 mol
The volume of KOH solution required can be calculated as:
V(KOH) = n(KOH) / M(KOH) = 0.13122 mol / 0.3544 mol/L = 0.3701 L
At equivalence, the total volume of the solution is:
V(total) = V(HC7H5O2) + V(KOH) = 0.1500 L + 0.3701 L = 0.5201 L
The concentration of the salt formed, KC7H5O2, is:
M(KC7H5O2) = n(KC7H5O2) / V(total) = n(HC7H5O2) / V(total) = 0.13122 mol / 0.5201 L = 0.2523 M
The pH at equivalence can now be calculated using the acid dissociation constant (Ka) of benzoic acid:
Ka = [H+][C7H5O2-] / [HC7H5O2]
pKa = -log(Ka) = -log(6.3 × 10^-5) = 4.20
At equivalence, the concentration of benzoic acid and benzoate ions are equal, so [HC7H5O2] = [C7H5O2-]. Let x be the concentration of H+ ions at equivalence. Then:
Ka = x^2 / (0.2523 - x)
Solving this equation for x gives:
x = sqrt(Ka × (0.2523 - x)) = sqrt(6.3 × 10^-5 × 0.2523) = 0.002532
Therefore, the pH at equivalence is:
pH = -log[H+] = -log(0.002532) = 2.60
Rounding to two decimal places, the pH at equivalence is 2.60.
What are the three main groups of macronutrients? (select three please)
minerals
proteins
fats
carboydrates
Answers
Answer:
protein, carbs, and fats.
Explanation:
Those are the three main sources of macronutrients.
(this isn't a chemistry, it's health)
Answer:
fats, carbohydrates and proteins
Explanation:
I don't know, I answered that on my test and it was correct
what mass of water (in grams) is produced by the reaction of 23.0 g of SiO2?
Answers
The mass of water produced by the reaction of the 23 g of \(SiO_2\) is 13.8 g.
The given chemical reaction;
\(4Hf (g) \ + \ SiO_2 (s) \ --> \ SiF_4(g) \ + \ 2H_2O(l)\)
In the given compound above, we can deduce the following;
molecular mass of \(SiO_2\) = 28 + (2 x 16) = 60 gmolecular mass of \(2H_2O\) = 2(18) = 36 g60 g of \(SiO_2\) --------- 36 g of water
23 g of \(SiO_2\) ------------- ? of water
\(mass \ of \ water = \frac{23 \times 36}{60} = 13.8 \ g \ of \ water\)
Thus, the mass of water produced by the reaction of the 23 g of \(SiO_2\) is 13.8 g.
"Your question is not complete, it seems to be missing the following information";In the reaction of the given compound, \(4Hf (g) \ + \ SiO_2 (s) \ --> \ SiF_4(g) \ + \ 2H_2O(l)\), what mass of water (in grams) is produced by the reaction of 23.0 g of SiO2?
Learn more here:https://brainly.com/question/13644576
A scientist observes the cells of a newly discovered animal under a
microscope. Which of the following cell parts is the scientist likely to find?
A. Cell membrane, chloroplasts, and a nucleus
B. Cell wall, cell membrane, and ribosomes
O C. Cell wall, chloroplasts, and lysosomes
D. Cell membrane, ribosomes, and a nucleus
Answers
Answer:
D
Explanation:
The rest have characteristics of a plant cell
Would a salt-cured egg be a physical or chemical change?
For a science experiment, I placed an egg in water that had three tablespoons of salt for one week. After 4 days, the egg cracked. At the end of the week, I took it out and cracked the egg. The egg yolk was now solid. This is also called salt-cured eggs. However, I'm not sure whether this is a physical or chemical change.
Answers
Answer:
I believe it is a physical change
Explanation:
The telltale sign of a chemical change is the formation of a new substance. They also usually involve the production of energy (heat, light, sound etc.)
............................................................................................................................
Answers
Answer:
............................................................................................................................
Explanation:
because ........................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................................