Answers
Answer:
You didn't provide any statements. If it is an option, art is a form of expression. Something created from imagination that expresses the thoughts and emotions of the artist.
The statement that best answers the question: What is art is that—Art is a form of visual language. Therefore, the correct option is A.
What is an art?Art is a diverse range of human activities and products that are created with the intention of expressing the imagination, emotions, or ideas, or to make a statement, in a visually appealing way.
Art can take many forms, including visual arts (such as painting, sculpture, and photography), performing arts (such as music, dance, and theater), and literary arts (such as poetry and prose).
It is typically seen as a form of communication and can be used to convey ideas, emotions, or political, social or cultural messages. It can also be used to simply evoke a response or emotion in the viewer, or to create a sense of beauty or aesthetic enjoyment. Therefore, the correct option is A.
Learn more about art, here:
https://brainly.com/question/8861182
#SPJ6
The question is incomplete, but most probably the complete question is,
Which of these statements best answers the question: What is art?
A. Art is a form of visual language.
B. Art is a form of written language.
C. Art is a form of spoken language.
D. Art is a form of dance language.
Related Questions
the beaker contains 0.2556 m h2so3 and the buret contains 0.3106 m naoh what happens to the conductivity during titration
Answers
Answer:
Before the equivalence point, conductivity is decreasing. After the equivalence point, conductivity is increasing
Explanation:
In solution H2SO3 produce H+ ions and SO3²⁻ ions. In the same way, NaOH produce Na⁺ and OH⁻ ions. The conductivity of a solution is directly proportional to the concentration of ions in a solution. During titration, you are adding more NaOH (That is, more Na⁺ and OH⁻ ions). But each moles of OH⁻ reacts with H⁺ ion producing H₂O. That means the moles of Na⁺ that you are adding = Moles of H⁺ are been consumed. The concentration of ions remains approximately constant. But, H⁺ ion conducts better than Na⁺ ion. That means before the equivalence point, conductivity is decreasing. But after the equivalence point you will add OH- ions in excess increasing ion concentration increasing the conductivity:
After equivalence point, conductivity is increasing.
A molecule has sp3d2 hybridization with 1 lone pair. ... The electron pair geometry of this molecule is: octahedral ... The geometry of this molecule is: octahedral . ... This molecule will have approximate bond angles of (If more than one bond angle is possible, separate each with a space.):
Answers
Answer:
electron pair geometry - octahedral
molecular geometry - square pyramidal
bond angle - < 90 degrees
Explanation:
According to Valence Shell Electron Pair Repulsion Theory (VSEPR), The shapes of molecules depend on the number of electron pairs on the outermost shell of the central atom in the molecule. Recall that electron pairs are always positioned as far apart in space as possible to minimize repulsion.
For a molecule in sp3d2 hybridization, the expected electron domain geometry is octahedral. However, the presence of a lone pair in the molecule distorts the electron pair geometry away from the expected octahedral shape giving a molecular geometry of square pyramidal and decreases the bond angle less than the expected 90 degrees.
Liquid nitrogen is kept at a temperature of -320 degrees. When liquid nitrogen is heated it quickly boils and turns into a gas. Which pair of pictures represent the change caused by adding heat to liquid nitrogen?
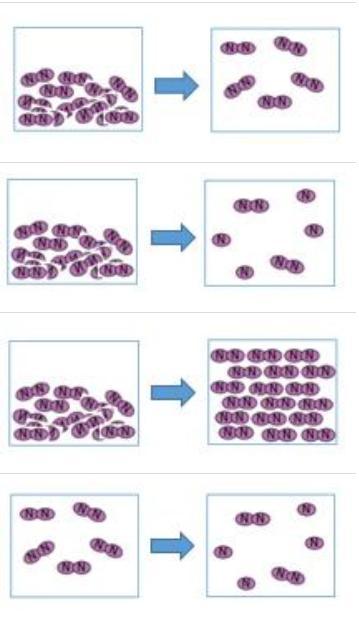
Answers
So when something is boiling it is moving faster meaning the molecules are more spread out and not condense like a solid
So the answer is the 2nd set
Hydrogen peroxide with a concentration of 3.0 percent (3.0 g of H2O2 in 100 mL of solution) is sold in drugstores for use as an antiseptic. For a 10.0-mL 3.0 percent H2O2 solution, calculate (a) the oxygen gas produced (in liters) at STP when the compound undergoes complete decomposition and (b) the ratio of the volume of O2 collected to the initial volume of the H2O2 solution.
Answers
Answer:
a) 0.099 L
b) 9.9
Explanation:
Now, given the equation for the decomposition of H2O2;
2H2O2(l) ------> 2H2O(l) + O2(g)
Mass of H2O2;
percent w/v concentration = mass/volume * 100
volume = 10.0-mL
percent w/v concentration = 3.0 percent
mass of H2O2 = x
3 = x/ 10 * 100
30 = 100x
x = 30/100
x = 0.3 g of H2O2
Number of moles in 0.3 g of H2O2 = mass/ molar mass
Molar mass of H2O2 = 34.0147 g/mol
Number of moles in 0.3 g of H2O2 = 0.3g/34.0147 g/mol
= 0.0088 moles
From the reaction equation;
2 moles of H2O2 yields 1 mole of oxygen
0.0088 moles of H2O2 = 0.0088 * 1/2 = 0.0044 moles of oxygen
If 1 mole of oxygen occupies 22.4 L
0.0044 moles of oxygen occupies 0.0044 * 22.4/1
= 0.099 L
b) initial volume of the H2O2 solution = 10 * 10-3 L
Hence, ratio of the volume of O2 collected to the initial volume of the H2O2 solution = 0.099 L/10 * 10-3 L = 9.9
-Convert 6.02 x 1020 formula units of MgCl₂ to mol of MgCl₂:
Answers
6.02 x \(10^{20\) formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
To convert formula units of MgCl₂ to moles of MgCl₂, we need to use Avogadro's number, which relates the number of formula units to the number of moles.
Avogadro's number (NA) is approximately 6.022 x 10^23 formula units per mole.
Given that we have 6.02 x 10^20 formula units of MgCl₂, we can set up a conversion factor to convert to moles:
(6.02 x 10^20 formula units MgCl₂) * (1 mol MgCl₂ / (6.022 x 10^23 formula units MgCl₂))
The formula units of MgCl₂ cancel out, and we are left with moles of MgCl₂:
(6.02 x 10^20) * (1 mol / 6.022 x 10^23) = 0.1 mol
Therefore, 6.02 x 10^20 formula units of MgCl₂ is equal to 0.1 moles of MgCl₂.
It's important to note that this conversion assumes that each formula unit of MgCl₂ represents one mole of MgCl₂. This is based on the stoichiometry of the compound, where there is one mole of MgCl₂ for every one formula unit.
Additionally, this conversion is valid for any substance, not just MgCl₂, as long as you know the value of Avogadro's number and the number of formula units or particles you have.
For more such questions on MgCl₂ visit:
https://brainly.com/question/26311875
#SPJ8
Which statement correctly describes gravity?
an attractive force that acts between all objects through contact
an attractive force that acts between all objects without contact
a repulsive force that acts between all objects through contact
a repulsive force that acts between all objects without contact
Answers
Answer:
I'd pick B, because gravity acts in between all object WITHOUT contact to each other. Gravity is all around us, we make contact with certain materials we use everyday, but objects don't contact to gravity. In certain terms, scientists are still not sure the REAL meaning of gravity, just how it works.
Explanation:
Pick B.
Answer:
I'd think it'd be B, because gravity acts between all objects without making contact.
Explanation:
Que símbolo tiene 25 protones,25 electrones y 27 neutrones
Answers
Define exothermic and endothermic. What are the mathematical signs of the internal energy and enthalpy when a process is exothermic?
Answers
Exothermic refers to chemical interactions that aerobic respiration. Combustion reactions release higher energy. Endothermic refers to atoms and molecules that either use or absorb reactive power.
What is an exothermic explanation?A chemical process known as an endothermic releases energy as heat or light. It is an endothermic reaction's opposite. Chemical equation expressed as reactants + products + energy. An reaction mechanism is one in which electricity is given off as light or warmth.
Exothermic example: What is it?A response is deemed to be exothermic if it produces heat while also undergoing a net decrease in basic enthalpy change. Samples include those type of combustion, iron rust, including water froze. Exothermic processes are those that discharge heat and energy into the surroundings.
To know more about exothermic visit:
https://brainly.com/question/13243759
#SPJ1
explain the role of sulphuric acid in limit test for iron
Answers
Consider the following reaction:
H2(g) + 2O2(g) --> 2H2O(g)
How many liters of H2O are produced when 15.7 liter of hydrogen are reacted with excess oxygen at STP?
Answers
Answer: 15.7 liters
Explanation: reaction is 2H2 + O2. -> 2H2O
Because all substance still are gases, volumes in
STP conditions are proportional to amount of moles.
Amount of water is same as Hydrogen. Also volume is same.
in the citric acid cycle (see the figure), beginning with one molecule of isocitrate and ending with fumarate, how many atp molecules can be made through substrate-level phosphorylation?
Answers
In the citric acid cycle , beginning with one of the molecule of isocitrate and ending with the fumarate, the ATP molecules can made through the substrate-level phosphorylation is one molecule.
In the citric acid cycle, the substrate level phosphorylation is the high energy of the phosphate group in the organic molecule. it transfers to the ADP to produces the ATP. it is the metabolic reaction and form the ATP molecules. The citric acid cycle is called as Krebs cycle also.
Thus, the number of ATP molecules form during the citric acid cycle that start with the one molecule of the isocitrate and end with the fumarate is the one molecule.
To learn more about citric acid cycle here
https://brainly.com/question/13030117
#SPJ4
Consider the reaction 2Al + 6HBr → 2AlBr3 + 3H2. If 12 moles of Al react with 12 moles of HBr, what is the limiting reactant?
Answers
Answer:
the limiting reactant is HBr
Explanation:
if you tried to make the products using 12 mol Al and 12 mol HBr, the HBr will run out first
Axel decided to start working out three times a week to stay healthy. Which type of disease prevention is being described?
Answers
Answer:
A
Explanation:
behavior modification
How do you think rocks form?
Answers
Answer:
Through gradual accumulation of sediments
Explanation:
How many different E2 products are expected in the reaction of 3-bromo-1,1-dimethylcyclohexane with NaOCH2CH3?
A) only 1
B) 2
C) 3
D) 4
Answers
if 0.30 moles of BF3 reacted with 0.90 moles of H2 which would be the limitung reactant
Answers
The limiting reactant, given that 0.30 moles of BF₃ reacted with 0.90 moles of H₂ is BF₃
How do I determine the limiting reactant?We'll begin by writing the balanced equation for the reaction. This is given below:
2BF₃ + 3H₂ → 2B + 6HF
From the balanced equation above,
2 moles of BF₃ reacted with 3 moles of H₂
With the above information, we shall determine the limiting reactant as follow:
From the balanced equation above,
2 moles of BF₃ reacted with 3 moles of H₂
Therefore,
0.3 mole of BF₃ will react with = (0.3 × 3) / 2 = 0.45 mole of H₂
From the above calculation, only 0.45 mole of H₂ out of 0.9 mole given, reacted with 0.3 mole of BF₃.
Thus, BF₃ is the limiting reactant
Learn more about limiting reactant:
https://brainly.com/question/11587316
#SPJ1
What was the unknown metal?
Answers
Answer:
is there a picture or something?
Explanation:
Explanation:
Your question is incomplete please update it.
A sample of ammonia, NH3, has a mass of 78.25 g. Calculate the number of ammonia molecules in the sample.
number of molecules:
Answers
There are approximately \(2.76 * 10^{24\) ammonia molecules in the given sample.
To calculate the number of ammonia molecules in the sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia \((NH_3)\) can be calculated by adding up the atomic masses of nitrogen (N) and hydrogen (H):
Molar mass of \(NH_3\) = (1 x atomic mass of N) + (3 x atomic mass of H)
= (1 x 14.01 g/mol) + (3 x 1.01 g/mol)
= 14.01 g/mol + 3.03 g/mol
= 17.04 g/mol
Now, we can calculate the number of moles of ammonia in the sample using the formula:
Number of moles = Mass of the sample / Molar mass
Number of moles = 78.25 g / 17.04 g/mol
≈ 4.5865 mol (rounded to four decimal places)
Finally, we can use Avogadro's number, which represents the number of particles (atoms, molecules, etc.) in one mole of a substance. Avogadro's number is approximately \(6.022 * 10^{23\) particles/mol.
Number of ammonia molecules = Number of moles x Avogadro's number
Number of ammonia molecules ≈ 4.5865 mol x (\(6.022 * 10^{23\) molecules/mol)
≈ \(2.76 * 10^{24\) molecules (rounded to two significant figures)
Therefore, the provided sample contains roughly \(2.76 * 10^{24\) ammonia molecules.
Learn more about moles on:
https://brainly.com/question/24748125
The number of ammonia molecules in the sample is approximately 2.764 x \(10^{24}\) molecules.
To calculate the number of ammonia molecules in a given sample, we need to use Avogadro's number and the molar mass of ammonia.
The molar mass of ammonia (NH3) is calculated as follows:
Molar mass of N = 14.01 g/mol
Molar mass of H = 1.01 g/mol
Total molar mass of NH3 = 14.01 g/mol + (3 * 1.01 g/mol) = 17.03 g/mol
Now, we can calculate the number of moles of ammonia in the sample:
Number of moles = Mass of sample / Molar mass of NH3
Number of moles = 78.25 g / 17.03 g/mol = 4.594 moles
Next, we use Avogadro's number, which states that there are 6.022 x \(10^{23}\) molecules in one mole of a substance.
Number of molecules = Number of moles * Avogadro's number
Number of molecules = 4.594 moles * 6.022 x \(10^{23}\) molecules/mol = 2.764 x \(10^{24}\) molecules
Therefore, there are approximately 2.764 x \(10^{24}\) ammonia molecules in the given sample of 78.25 g.
Know more about Avogadro's number here:
https://brainly.com/question/1513182
#SPJ8
What does the term "thermometer" mean?
Answers
Answer:A thermometer is a tool that measures temperature — how hot or cold something is. Thermometers are used to see if you have a fever or tell you how cold it is outside.
Explanation:
Answer: A Device That Measures Temperature.
Explanation:
Which is the correct set of products for the acid-base neutralization reaction between hydrochloric acid and sodium hydroxide?
Answers
Answer: I think the answer is C. NaCl and H2O
Explanation: I’m not sure tho
Answer:
C) - Water and a Salt
Explanation:
Because Water and a Salt are the products of a neutralization reaction between an acid and a hydroxide base.
What is the minimum number of dots an atom can show
Answers
Answer:
By going through the periodic table, we see that the Lewis electron dot diagrams of atoms will never have more than eight dots around the atomic symbol.
Explanation:
Search :)
In Chemistry we look at the composition and the_
change Y
of matter
Answers
Explanation:
A pure substance is a form of matter that has a constant composition and properties that are constant throughout the sample. Mixtures are physical combinations of two or more elements and/or compounds
Which of the following is a correctly written chemical equation that demonstrates the conservation of mass?
Mg + HC1
→
H
2
+
MgC
1
2
Mg + HC1 → H 2 + MgC 1 2
H
2
O
+
C
O
2
→
H
2
C
O
3
H 2 O + C O 2 → H 2 C O 3
KC
1
O
3
→
KC1 +
O
2
KC 1 O 3 → KC1 + O 2
H
2
+
O
2
→
H
2
O
H 2 + O 2 → H 2 O
Answers
if I'm not mistaking it would be the second option
Which bonds form in the reaction shown in the diagram? 2 2H, + 0 H-H O=0 H-H → 2H 0 H-0-H H-OH A. The bonds between the two hydrogen atoms and between the two oxygen atoms B. The bonds between the two hydrogen atoms C. The bonds between the oxygen and hydrogen atoms D. The bonds between the two oxygen atoms

Answers
The water molecule is formed by the covalent bonding between oxygen and hydrogen atoms. Therefore, option C is correct.
What is covalent bonding ?Covalent bonding is a type of chemical bonding that involves the sharing of electrons between two atoms. In covalent bonding, the two atoms share a pair of electrons to fill their outermost electron shell and form a stable molecule.
This type of bonding usually occurs between non-metal atoms, which have a high electronegativity and tend to attract electrons strongly. In a covalent bond, the shared electrons are attracted to the positively charged nuclei of both atoms, creating a strong bond.
The strength of the bond depends on the number of shared electrons and the distance between the nuclei. Covalent bonds can be either polar or nonpolar, depending on the electronegativity difference between the two atoms.
In water the bond is formed between oxygen atom and two hydrogen atoms. Hence, C is correct.
Find more on covalent bonding:
https://brainly.com/question/12661797
#SPJ7
Which of the following factors contribure
to smog problems?
a. high numbers of automobiles
b. lots of sunlight
c. mountains surrounding urban areas
d. all of the above
Answers
Ground level ozone is created when sunlight reacts with certain chemicals that come from sources of burning fossil fuels, such as factories or car exhaust. When particles in the air combine with ozone, they create smog. Smog is a type of air pollution that looks like smoky fog and makes it difficult to see. Additionally, Cities located in basins surrounded by mountains may have smog problems because the smog is trapped in the valley and cannot be carried away by wind
What do you suppose is the reason and what do you need to do.
Answers
Answer:
You have to add many things
Explanation:
for example u can add why and how also what ur point is
In the chemical equation 2Mg(s) + O2(g) ? 2MgO(s),
a Mg represents the product magnesium.
b. the reaction yields magnesium.
c. Mg represents the reagent magnesium.
d. O, represents the product oxygen gas.
Answers
In the reaction; 2Mg(s) + O2(g) ------> 2MgO(s), Mg represents the reagent magnesium.
What is a chemical reaction?The term chemical reaction has to do with the interaction between compounds which are called reactants to yield a new compound which is called the product.
In the reaction; 2Mg(s) + O2(g) ------> 2MgO(s), Mg represents the reagent magnesium.
Learn more about reaction:https://brainly.com/question/17434463
#SPJ1
Based on this passage, the term "mechanical disintegration" means
breaking into small pieces
separation of solid and liquid
evaporation of gases in talus
cultivation of grains
Answers
Mechanical disintegration means breaking into small pieces (option A).
What is mechanical digestion?Digestion is the process occuring in the gastrointestinal tract, by which food is converted into substances that can be utilized by the body.
Digestion can, however, be mechanical/physical or chemical/enzymatical. The mechanical digestion involves the breaking down of food into smaller pieces by teeth.
Therefore, according to this question, there is no passage, however, the meaning of mechanical disintegration can be easily detected in biology.
Learn more about mechanical digestion at: https://brainly.com/question/15457673
#SPJ1
A solution contains 0.470 mol of isopropanol (C3H7OH) dissolved in 3.320 mol of water
a)What is the mole fraction of isopropanol?
b)What is the mass percent of isopropanol?
c)What is the molality of isopropanol?
Answers
A. The mole fraction of isopropanol in the solution is 0.124
B. The mass percent of isopropanol is 12.4%
C. The molality of isopropanol is 7.86 M
A. How to determine the mole fraction of isopropanolMole of isopropanol = 0.470 moleMole of water = 3.320 mole Total mole = 0.470 + 3.32 = 3.79 mole Mole fraction of isopropanol =?Mole fraction = mole / total mole
Mole fraction of isopropanol = 0.470 / 3.79
Mole fraction of isopropanol = 0.124
B. How to determine the percentage of isopropanolMole of isopropanol = 0.470 moleTotal mole = 3.79 mole Percentage of isopropanol =?Percentage = (mole / total mole) × 100
Percentage of isopropanol = (0.470 / 3.79) × 100
Percentage of isopropanol = 12.4%
C. How to determine the molality Mole of isopropanol = 0.470 moleMole of water = 3.320 mole Mass of water = 3.320 × 18 = 59.76 g = 59.76 / 1000 = 0.05976 KgMolality of isopropanol =?Molality = mole / Kg of water
Molality of isopropanol = 0.47 / 0.05976
Molality of isopropanol = 7.86 M
Learn more about Molality:
https://brainly.com/question/4251997
Determining Overall Reaction Rate Laws from Experimental Data. Butadiene, C4H6, dimerizes in a Diels-Alder condensation reaction to yield a substituted cyclohexene, C8H12. Given the data on the 500 K gas phase reaction below, answer the following questions.
Time (s)
Total Pressure (torr)
C4H6 Partial Pressure (torr)
0
1250
1250
750
1160
1070
1500
1090
930
2460
1020
790
3425
950
650
4280
920
590
5140
900
550
6000
880
510
7500
850
450
9000
820
390
10500
810
370
a. Write the balanced chemical reaction and the overall differential rate law assuming the overall reaction rate is only a function of C4H6 concentration (CC4H6), reaction rate constant (k), and reaction rate order (a).
b. Using relative rates of reaction, what is the differential rate law for C4H6?
c. Plot the linearized form of CC4H6 as a function of time, assuming the following reaction rate orders. Each order should be a separate graph and include the linear trendline equation and R2 value.
i. Zero order
ii. First order
iii. Second order
d. From the graphs in part (c), what is the experimental rate order of this reaction?
e. What is the experimental reaction rate constant, k, including units?
Answers
a) d[C₈H₁₂]/dt = k * [C₄H₆]ᵃ, b) d[C₄H₆]/dt = -k * [C₄H₆]ᵃ . c) i. Zero order: ln([C₄H₆]0/[C₄H₆]) = -kt, [C₄H₆] = [C₄H₆]0 * e⁽⁻kt⁾, ii. First order:, ln([C₄H₆]/[C₄H₆]0) = -kt, [C₄H₆] = [C₄H₆]0 * e⁽⁻kt⁾, iii. Second order:, 1/[C₄H₆]=kt + 1/[C₄H₆]0, [C₄H₆] = 1 / (kt + 1/[C₄H₆]0), d) From the graphs in part (c), the experimental rate order of this reaction is first order. e) The experimental reaction rate constant, k, can be determined from the slope of the first order plot and has units of 1/s.
The balanced chemical reaction for the Diels-Alder condensation of butadiene (C₄H₆) to yield a substituted cyclohexene (C₈H₁₂) is C₄H₆+ C₄H₆-> C₈H₁₂. The overall differential rate law is d[C₈H₁₂]/dt = k * [C₄H₆]ᵃ, where k is the reaction rate constant and a is the reaction rate order. The differential rate law for C₄H₆is d[C₄H₆]/dt = -k * [C₄H₆]ᵃ. The rate order is determined from the linearized form of the data, which shows that the reaction is first order with a reaction rate constant k having units of 1/s.
a. The balanced chemical reaction and the overall differential rate law assuming the overall reaction rate is only a function of C₄H₆ concentration (CC₄H₆), reaction rate constant (k), and reaction rate order (a) is given by:
C₄H₆ + C₄H₆ -> C₈H₁₂
d[C₈H₁₂]/dt = k * [C₄H₆]ᵃ
b. The differential rate law for C₄H₆ is given by:
d[C₄H₆]/dt = -k * [C₄H₆]ᵃ
c. The linearized form of CC₄H₆ as a function of time for each reaction order:
i. Zero order:
ln([C₄H₆]0/[C₄H₆]) = -kt
[C₄H₆] = [C₄H₆]0 * e⁽⁻kt⁾
ii. First order:
ln([C₄H₆]/[C₄H₆]0) = -kt
[C₄H₆] = [C₄H₆]0 * e⁽⁻kt⁾
iii. Second order:
1/[C₄H₆] = kt + 1/[C₄H₆]0
[C₄H₆] = 1 / (kt + 1/[C₄H₆]0)
d. From the graphs in part (c), the experimental rate order of this reaction is first order.
e. The experimental reaction rate constant, k, can be determined from the slope of the first order plot and has units of 1/s.
Learn more about rate law here: brainly.com/question/30379408
#SPJ4