Which one show more vapour pressure?
A. alcohol B. gasoline C. water D. both a & b
Answers
Related Questions
Which of the following is NOT a common property of water?
o Water has high surface tension.
o Water expands when it freezes.
o Ice is less dense than water.
o Water has low heat of vaporization.
Answers
Answer:
A = water has high surface tension
the answer is a hope this helps and plz give me brainlist :)
The temperature of a piece of copper with a mass of 95.4g increases from 25 degrees Celsius to 48.0 degrees Celsius when the metal absorbs 849 J of heat. What is the specific heat capacity of copper?
Answers
Answer:
The answer is 3.87 J/g°C
Explanation:
Here is the equation we are going to use:
\(C=\frac{q}{mT}\)
C= specfic heat in J/g°C
q = heat in joules (J)
m = mass in grams (g)
T = change in temperature
Here is what is given:
q = 849 J
m = 95.4 g
T = 48.0 - 25.0 = 23°C
Find:
Specific heat capacity in J/g
The first thing we are going to do is plug everything into the equation:
\(C = \frac{849J}{(9.54g)(23degreesC)}\)
Then we are going to solve for C
\(C = \frac{849J}{219.42gC} = 3.87 J/gCelsius\)
Hope this helps!
Answer:
The answer is 3.87 J/gC
0.10 moles of sodium sulfate is dissolved into 12 mL of solution. What is the molar concentration of the solution?
Your answer should have two significant figures (round your answer to one decimal place).
Answers
the molar concentration should be atleast 5mL off the solution since its disolved
Answer:
8.3
Explanation:
Convert mL to L.
12 mL×1 L1000 mL=0.012 L
Solve for molarity.
Molarity=moles of solute
liters of solution=0.10 mol0.012 L=8.3 M
Mass = 35g
Density = 5 g/cm3
∙What is the Volume?
Answers
Answer:
7cm3
.........
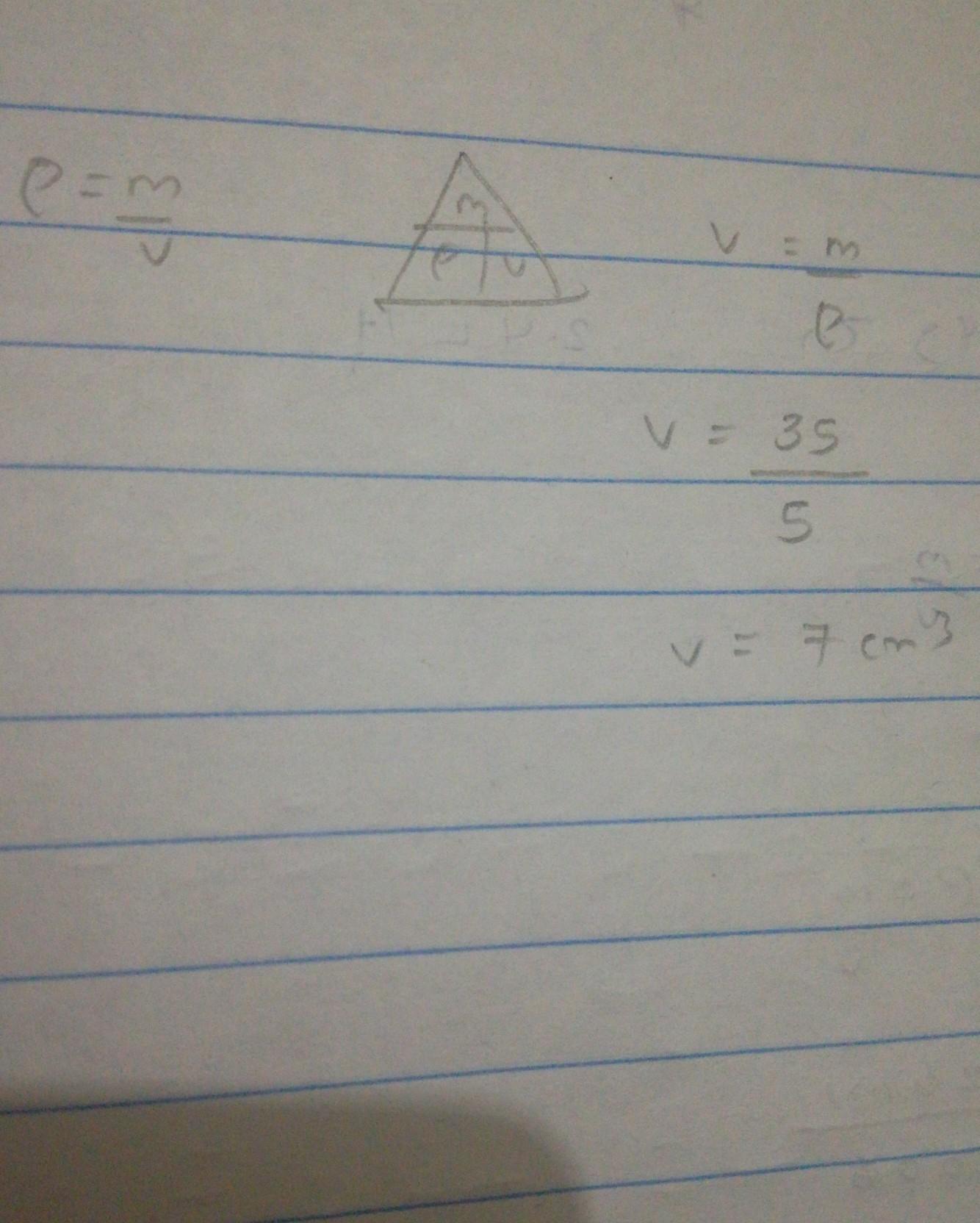
1
2
3
4
5
6
7
8
9
10
51:25
Current vs. Force
What type of relationship does this graph show?
0.00300
0.00250
c
O a negative relationship
an inverse relationship
O a non-linear relationship
O a direct relationship
0.00200
Force (N)
0.00150
0.00100
0.00050
1.40 1.60
0.20 0.40 0.60 0.80 1.00 1.20
Current (A)
Answers
Answer:
this is not good
Explanation:
alost got the asnwe
s
831.8 mL of gas is at 49.2 C. It is compressed to a volume of 79 mL. What is the new temperture. Express your answer in Kelvin.
Answers
The temperature T2 is 343.91 K. We would need additional information about the pressures at the initial and final states of the gas to calculate the final temperature.
What is Temperature?
Temperature is a measure of the average kinetic energy of the particles in a substance, such as a gas, liquid, or solid. It is commonly associated with the sensation of hotness or coldness, and is typically measured in units such as Celsius (°C), Fahrenheit (°F), or Kelvin (K).
To find the new temperature of the gas after compression, we can use the combined gas law, which relates the initial and final states of a gas undergoing changes in pressure, volume, and temperature.
The combined gas law formula is given as:
(P1 * V1) / T1 = (P2 * V2) / T2
P1 = pressure of the gas at the initial state (unknown)
V1 = initial volume of the gas = 831.8 mL
T1 = initial temperature of the gas = 49.2 + 273.15 K (converting Celsius to Kelvin)
P2 = pressure of the gas at the final state (unknown)
V2 = final volume of the gas = 79 mL
T2 = final temperature of the gas (unknown)
We need to solve for T2, the final temperature of the gas.
Rearranging the formula to solve for T2, we get:
T2 = (P2 * V2 * T1) / (P1 * V1)
Now we can plug in the given values and solve for T2:
T2 = (P2 * 79 * (49.2 + 273.15)) / (P1 * 831.8)
T2 = (P2 * 79 * (49.2 + 273.15)) / (P1 * 831.8)
T2 = (2.0 atm * 79 * (49.2 + 273.15 K)) / (1.5 atm * 831.8)
T2 = 343.91 K
Therefore, the temperature T2 is 343.91 K.
Learn more about Temperature from the given link
https://brainly.com/question/26866637
#SPJ1
277K is the new temperature if 831.8 mL of gas is at 49.2 C and is compressed to a volume of 79 mL.
What is the definition of the ideal gas law?
The rule that states that the sum of the absolute temperature of the gas and the universal gas constant is equal to the product of the pressure and volume of a single gram of an ideal gas.
The phrase "ideal gas" describes a fictitious gas made up of molecules that adhere to the following principles: No attraction or repellence exists between the molecules of ideal gases. The sole interaction between molecules of an ideal gas would be an elastic collision when they collided or an elastic collision with the container walls.
(P1 * V1) / T1 = (P2 * V2) / T2
V1 = 831.8 mL
T1 = 49.2 + 273.15 K
V2 = 79 mL
T2 = final temperature of the gas
To solve for T2, the final temperature of the gas.
T2 = ( V2 * T1) / ( V1)
T2 = 79*322.35/831.8
T2 = 277K
To learn more about Temperature use:
brainly.com/question/26866637
#SPJ1
what happens to the gaseous phase of water when it reaches a metal lid
Answers
Answer:
When the gaseous phase of water reaches a metal lid it condenses to form water.
Explanation:
The gaseous phase of water is at a higher temperature than the temperature of a metal lid. Thus when it comes in contact with the cold lid it loses heat and gets converted to the liquid phase.
When water vapor loses temperature, it undergoes a process called condensation, which results in its conversion to a liquid state. This process is essentially the opposite of vaporization. Typically, condensation occurs when a vapor is compressed or cooled to its saturation limit, causing the molecular density within the gas phase to reach its maximum threshold.
To know more about condensation,
https://brainly.com/question/30629848
In heterogeneous mixtures, you would be able to see the two or more substances that are in the mixture?
Answers
In heterogeneous mixtures, you would indeed be able to see the two or more substances that make up the mixture. This is because in a heterogeneous mixture, the different substances are not evenly distributed throughout the mixture. Instead, they are typically visible as distinct phases or particles.
For example, if you mix sand and water together, you can clearly see the sand particles floating in the water. Similarly, if you mix oil and vinegar together, you can see the separate layers of oil and vinegar.
In these cases, the substances do not dissolve or combine completely, allowing us to visually observe the different components. This is different from a homogeneous mixture, where the substances are evenly mixed and not visibly separate.
To know more about heterogeneous mixtures visit:-
https://brainly.com/question/24898889
#SPJ11
Write a balanced Al(s), Ba(s), Ag(s), and Na(s) for the synthesis reaction of Br2(g).
Answers
The synthesis reaction of Br2(g) with Al(s), Ba(s), Ag(s), and Na(s) are as follows:Br2(g) + 2 Al(s) → 2 AlBr3(s)3 Br2(g) + Ba(s) → BaBr6(s)2 Ag(s) + Br2(g) → 2 AgBr(s)2 Na(s) + Br2(g) → 2 NaBr(s)
Balanced equation for the synthesis reaction of Br2(g) with Al(s), Ba(s), Ag(s), and Na(s)Br2(g) + 2 Al(s) → 2 AlBr3(s) 3 Br2(g) + Ba(s) → BaBr6(s) 2 Ag(s) + Br2(g) → 2 AgBr(s) 2 Na(s) + Br2(g) → 2 NaBr(s)The synthesis reaction of Br2(g) can be carried out using different metals such as Al(s), Ba(s), Ag(s), and Na(s). The balanced chemical equation for the reaction will be based on the type of metal used. However, all of the reactions will produce a metal bromide salt.The first equation represents the reaction of Br2(g) with aluminum. This reaction results in the formation of aluminum tribromide salt. The balanced chemical equation for the reaction is as follows:Br2(g) + 2 Al(s) → 2 AlBr3(s)The second equation represents the reaction of Br2(g) with barium. This reaction results in the formation of barium hexabromide salt. The balanced chemical equation for the reaction is as follows:3 Br2(g) + Ba(s) → BaBr6(s)The third equation represents the reaction of Br2(g) with silver. This reaction results in the formation of silver bromide salt. The balanced chemical equation for the reaction is as follows:2 Ag(s) + Br2(g) → 2 AgBr(s)The fourth equation represents the reaction of Br2(g) with sodium. This reaction results in the formation of sodium bromide salt. The balanced chemical equation for the reaction is as follows:2 Na(s) + Br2(g) → 2 NaBr(s)In conclusion, the balanced chemical equations for
For more such questions on chemical equation
https://brainly.com/question/11904811
#SPJ8
After the correct formula for a reactant in an equation has been written, the:
A. Subscripts are adjusted to balance the equation
B. Formula should not be changed
C. Same formula must appear as the product
D. Symbols in the formula must not appear on the product side of the equation
Answers
Once a reactant's correct formula has been placed in an equation, the A. The equation is balanced by adjusting the subscripts.
The correct formula for a reactant in a chemical equation must be written first, and then the subscripts are adjusted to balance the equation. Balancing the equation means making sure that the same number of each type of atom appears on both the reactant and product sides of the equation. This can be done by adding coefficients in front of the formulas as necessary. Once the equation is balanced, the formula should not be changed. The same formula must appear on both the reactant and product sides of the equation, but the coefficients may be different. The symbols in the formula must not appear on the product side of the equation unless the formula has been balanced first.
Learn more about atoms here:
https://brainly.com/question/1566330
#SPJ4
Considere la siguiente reacción: H, (g) +1, (a) = 2 HI (9). K, para la reacción es 54.3 a 430°C. Si se coloca H, 0.00623M, 0.00414M y HI 0.0224M en un recipiente, calcule las concentraciones de las especies luego de alcanzar equilibrio.
Answers
Answer:
[HI] = 0.0255M
[H₂] = 0.00466M
[I₂] = 0.00257M
Explanation:
Para la reacción:
H₂(g) + I₂(g) ⇄ 2HI(g)
La constante de equilibrio, K, se define como:
54.3 = [HI]² / [H₂] [I₂]
Donde cada concentración [] será la concentración en equilibrio para cada especie
Para saber la dirección del equilibrio definiremos Q como:
Q = [HI]² / [H₂] [I₂]
Donde las concentraciones [] serán las concentraciones actuales de cada gas
Reemplazando:
Q = [0.0224M]² / [0.00623M] [0.00414M]
Q = 19.5
Como Q<K, la reacción se desplazará hacia la derecha produciendo más [HI]. Así, las concentraciones en equilibrio serán:
[HI] = 0.0224M +2X
[H₂] = 0.00623M - X
[I₂] = 0.00414 - X
54.3 = [0.0224M +2X]² / [0.00623M - X] [0.00414M - X]
54.3 = 0.00050176 + 0.0896 X + 4 X² / 0.0000257922 - 0.01037 X + X²
0.00140052 - 0.563091 X + 54.3 X² = 0.00050176 + 0.0896 X + 4 X²
0.00089876 - 0.652691 X + 50.3 X² = 0
Resolviendo la ecuación cuadrática:
X = 0.001566M → Solución verdadera
X = 0.01141M → Falsa solución. Produciría concentraciones negativas
Reemplazando:
[HI] = 0.0224M +2*0.001566M
[H₂] = 0.00623M - 0.001566M
[I₂] = 0.00414 - 0.001566M
[HI] = 0.0255M
[H₂] = 0.00466M
[I₂] = 0.00257M
Siendo estas últimas, las concentraciones de las especies luego de alcanzar el equilibrio.
YOU ARE AMAZING AND YOU ARE SO IMPORTANT HAVE A GOOD DAY!
Answers
Answer:
Thx Have a fantastic day! :)
Explanation:
What does control group mean
Answers
1. what will be the mass of 1 atom of C-12 in grams?
2. What is the difference b/w molality&molarity.
3. Hydrogen gas is prepared in the laboratory by reacting dilute HCl with granulated zinc.
The following reaction takes place:
Zn + 2HCL ----› ZnCL2 +H2
Calculate the volume of hydrogen gas liberated at STP when 32.65g of zinc reacts with HCl. 1 mole of a gas occupies 22.7Litre volume at STP; Atomic mass= zn is 65.3u/amu.
4. the density of a 3 molal solution of NaOH is 1.110g m/l. calculate the molarity of the solution.
Answers
\({ \qquad\qquad\huge\underline{{\sf Answer}}} \)
Here we go ~
Question 1Mass of 1 mole C - 12 atom = 12 g
So, mass of 1 carbon - 12 atom = ( 12 / 1 mole ) g
that is :
\(\qquad \sf \dashrightarrow \: \cfrac{12}{6.022 \times 10 {}^{23} } \: \: g\)
[ since 1 mole = 6.022 × 10²³ ]
\( \qquad \sf \approx2 \times 10 {}^{ - 23} \: \: g\)
Question 2Molarity :
Molarity is defined as " The number of moles of solute present in per litre of solution "
Denoted as M = [ moles / litre ]change in temperature can cause change in Molarity, as the volume of solution varies with temperature. change in pressure can also cause change in Molarity, as volume is affected by pressure as well.Molality :
Molality is defined as " Number of moles of solute present per kg mass of solvent "
Denoted as m = [ moles / kg ]It isn't affected by any external factors like temperature or pressure, as mass of solvent is constant. Question 3As per the given reaction ~
\(\qquad \sf \dashrightarrow \: Zn + 2\:H Cl \rightarrow ZnCl_2 + H_2\)
32.65 g of zinc reacted,
[ Number of moles of zinc reacted = mass of zinc reacted divided by its formula Weight ]
\(\qquad \sf \dashrightarrow \: number \: \: of \: \: moles = \cfrac{32.65}{65.3} \: \: mol\)
\(\qquad \sf \dashrightarrow \: number \: \: of \: \: moles = \cfrac{1}{2} \: \: mol\)
so, we can say that " half mole Zinc reacted with 1 mole of HCl to form half mole of Zinc chloride and half mole of Hydrogen gas "
And we already know that 1 mole of any gas occupies 22.7 litre volume at STP.
So, volume of Hydrogen gas Liberated :
\(\qquad \sf \dashrightarrow \: \cfrac{1}{2} \times 22.7\)
\(\qquad \sf \dashrightarrow \: 11.35 \: \: litres\)
Question 4The relationship between Molarity and molality can be expressed as :
\(\qquad \sf \dashrightarrow \: M =\cfrac{ 1000(m \times d)}{1000+(m \times F)}\)
Terms :
M = Molarity = ?m = molality = 3 molald = density = 1.110 g/lF = formula weight/molar mass = 40 g\(\qquad \sf \dashrightarrow \: M =\cfrac{ 1000(3 \times 1.110)}{1000+(3 \times 40)}\)
\(\qquad \sf \dashrightarrow \: M =\cfrac{ 1000( 3.330)}{1000+120}\)
\(\qquad \sf \dashrightarrow \: M =\cfrac{ 3330}{1120}\)
\(\qquad \sf \dashrightarrow \: M =2.973 \: mol \: l {}^{ - 1} \)
How many times more acidic is a pH of 2 than a PH of 7?
Answers
Answer: 10 times more
Explanation:
The AP Biology teacher is measuring out 638.0 g of dextrose (C6H12O6) for a lab. How many moles of dextrose is this equivalent to?
Answers
The AP Biology teacher is measuring out 638.0 g of dextrose (C6H12O6) for a lab the moles of dextrose is this equivalent to is 3.6888 moles.
What are moles?A mole is described as 6.02214076 × 1023 of a few chemical unit, be it atoms, molecules, ions, or others. The mole is a handy unit to apply due to the tremendous variety of atoms, molecules, or others in any substance.
To calculate molar equivalents for every reagent, divide the moles of that reagent through the moles of the restricting reagent. The calculation is follows:
655/12 x 6 + 12+ 16 x 6 = 655/ 180 = 3.6888 moles.Read more about moles:
https://brainly.com/question/24322641
#SPJ1
In which compound do atoms form bonds by sharing electrons ?
Answers
Answer:
-a covalent bond.
Explanation:
For the equilibrium
2H2S(g) ⇋ 2H2(g) + S2(g) Kc = 9 .0X 10-8 at 700°C
the initial concentrations of the three gases are 0.300 M H2S, 0.300 M H2, and 0. 1 50 M S2' Determine the equilibrium concentrations of the gases.
Answers
Answer:
Equilibrium concentrations of the gases are
\(H_2S=0.596M\)
\(H_2=0.004 M\)
\(S_2=0.002 M\)
Explanation:
We are given that for the equilibrium
\(2H_2S\rightleftharpoons 2H_2(g)+S_2(g)\)
\(k_c=9.0\times 10^{-8}\)
Temperature, \(T=700^{\circ}C\)
Initial concentration of
\(H_2S=0.30M\)
\(H_2=0.30 M\)
\(S_2=0.150 M\)
We have to find the equilibrium concentration of gases.
After certain time
2x number of moles of reactant reduced and form product
Concentration of
\(H_2S=0.30+2x\)
\(H_2=0.30-2x\)
\(S_2=0.150-x\)
At equilibrium
Equilibrium constant
\(K_c=\frac{product}{Reactant}=\frac{[H_2]^2[S_2]}{[H_2S]^2}\)
Substitute the values
\(9\times 10^{-8}=\frac{(0.30-2x)^2(0.150-x)}{(0.30+2x)^2}\)
\(9\times 10^{-8}=\frac{(0.30-2x)^2(0.150-x)}{(0.30+2x)^2}\)
\(9\times 10^{-8}=\frac{(0.30-2x)^2(0.150-x)}{(0.30+2x)^2}\)
By solving we get
\(x\approx 0.148\)
Now, equilibrium concentration of gases
\(H_2S=0.30+2(0.148)=0.596M\)
\(H_2=0.30-2(0.148)=0.004 M\)
\(S_2=0.150-0.148=0.002 M\)
THIS IS FOR SCIENCE PLS HELP ME WRITE MY PROMPT How does the human population and human activity affect Earth?
Answers
calculate the relative molecular mass of sodium trioxonitrate (V) NaNO3 [Na = 23,O=16,Ca= 40, H=1,N= 14]
Answers
The relative molecular mass of sodium trioxonitrate, NaNO₃ is 85 g/mol.
What is molecular mass?The molecular mass of a substance is the sum of all atomic masses of all atoms in a molecule. It is measured in Dalton.
The compound is NaNO₃ and the atomic mass of all atoms are given below:
Atomic Mass of sodium (Na) = 23 g
Atomic Mass of nitrogen (N) = 14 g
Atomic Mass of oxygen (O) = 16 g
Now add the atomic masses of atoms of the molecules:
Molecular mass of = 23 +14 +16(3) = 85 g/mol.
Thus, the relative molecular mass of sodium trioxonitrate is 85 g/mol.
To learn more about molecular mass, refer to the link:
https://brainly.com/question/18446366
#SPJ1
What limitations would you encounter if you attempted to simultaneously measure the position and the momentum of a proton?.
Answers
The limitations would you encounter if you attempted to simultaneously measure the position and momentum of a proton is the uncertainty in its momentum multiplied by the uncertainty in its position is always greater than or equal to h/4π.
Understanding Heisenberg's Theory
This Heisenberg Uncertainty Principle played an important role in 20th century physics, especially the development of quantum mechanics. The principle of uncertainty influences the development of modern philosophy.
Heisenberg received the Nobel Prize in Physics in 1932. Throughout his life, Heisenberg produced many papers, including the Principles of Quantum Theory Physics (1930). Cosmic Radiation (1946), Physics and Philosophy (1958), and Introduction to the Unified Theory of Elementary Particles (1967).
The uncertainty theory developed by Heisenberg was challenged by Einstein. According to this theory, the more accurately we determine the position of an object, the less accurate its momentum (or velocity), and vice versa. So, we cannot determine the location of objects accurately. In other words, objects have the possibility of being anywhere.
Einstein said that the theory is nonsense. Until the end of his life he opposed Heisenberg's Uncertainty Theory. Einstein opposed because he did not believe in the Uncertainty Theory which states that the position of the moon is uncertain.
Learn more about uncertainty theory at https://brainly.com/question/29392632.
#SPJ4
suppose a biochemist has 10 ml of a 1.0 m solution of a compound with two ionizable groups at a ph of 8.00. she adds 10.0 ml of 1.00 m hcl, which changes the ph to 3.20. the value of one of the groups is 3.8 and it is known that is between 7 and 10. what is the exact value of ?
Answers
To determine the exact value of pK2 in this case, we can use the relationship between pH and pK, which states that the pH of a solution is equal to the pK of the acid with the lowest concentration when the acid and its conjugate base are in equilibrium.
Here, we know that the pH of the solution dropped from 8.00 to 3.20 after the addition of HCl. We can assume that the second group is now in the acid form since we know that the first ionizable group has a pK of 3.8, and the second group (pK2) is between 7 and 10, we can assume that the second group is now in the acid form.
We can use the relationship between pH and pK to calculate the exact value of pK2:
pH = pK2 = pKw / [H+] = 14.00 - log([H+])
3.20 = 14.00 - log([H+])
Solving for [H+], we find that [H+] = 1.0 x 10^-3.2
Now we can find the value of pK2 as:
pK2 = 14.00 - log(1.0 x 10^-3.2) = 14.00 - (-3.2) = 14.00 + 3.2 = 17.2
Therefore, the exact value of pK2 is 17.2.
Learn more about Equilibrium here: https://brainly.com/question/28527601
#SPJ4
Help!
Which of the following can scientists NOT interpret by examining fossils?
A. How earth's environment has changed overtime.
B. How plants and animals have changed overtime.
C. The age of certain layers of rocks. D. How the pull of gravity has changed.

Answers
Blood type in humans is controlled by ____
alleles.
a, three
b. two
c. one
d. four
Answers
Answer:
A: three
Explanation:
ITEM BANK: Move to Bottom
Balance
Measured in Newtons
Measured in grams
Property of matter
Spring Scale
Weight
Both
Mass
Cugan 100
drag and drohte
Answers
Answer:
The scale reads the tension in the string. The tension in the string is 100 N. This is the force the string must exert up on either of the 100-N weights at either end of the string.
Nothing is moving, nothing is accelerating, so the net force on the spring is zero. Likewise, the net force on either of the 100-N weights is also zero. But that is another question. The spring scale does not measure the net force. The spring scale simply measures the tension, the magnitude of the force exerted by the string.
Explanation:
Acetylene, C2H2, burns according to the following reaction: C2H2 5O2 --> 4CO2 2H2O. Suppose 1.20 g of C2H2 is mixed with 3.50 g of O2 in a closed, steel container, and the mixture is ignited. What substances will be found in the mixture left when the burning is complete
Answers
C2H2 will be left when the burning is complete.
The equation of the reaction is; 2C2H2 + 5O2 --> 4CO2 + 2H2O
The number of moles of C2H2 reacting is = 1.20 g/26 g/mol = 0.046 moles
The number of moles of O2 is = 3.50 g/32 g/mol = 0.109 moles
Since;
2 mole of C2H2 reacts with 5 moles of O2
x moles of C2H2 reacts with 0.109 moles of O2
x = 2 mole × 0.109 moles/5 moles
x = 0.044 moles of C2H2.
It then follows that C2H2 is the reactant in excess so C2H2 will be left when the burning is complete.
Learn more: https://brainly.com/question/9743981?
when a gas is collected over water, is the gas pure? why or why not? how can the partial pressure of the collected gas be determined?
Answers
When a gas is collected over water, the gas is not pure because it is mixed with vapor from the evaporation process of the water.
Partial pressure is the pressure that a gas would have if it took up the entire volume that the mixture of gases currently occupies.
Gas is not pure when it is collected over water because it contains water molecules. Dalton's Law of Partial Pressures can be used to calculate the partial pressure of the collected gas.
Following the determination of the total pressure, the pressure of the water vapor is calculated by consulting a reference table for that particular temperature. By deducting the pressure of the water vapor from the total pressure, one can calculate the partial pressure of the collected gas.
Learn more about partial pressure here: https://brainly.com/question/14119417
#SPJ4
Please pray for healing and my name is Fairouz

Answers
Answer:
I hope everything gets better
Explanation:
Answer:
I hope you are better now
A pool float was filled with air to capacity in the middle of a hot day. The next morning the pool float looked different yet no gas escaped or entered the float. Describe what you think the float looked like in the morning and why it occurred.
Answers
Answer:
The pool float looked would have looked deflated the next morning.
Explanation:
When temperature decreases the volume decreases
Answer:
The pool float most likely looked a little deflated or pushed in
Explanation:
The pool float most likely looked a little deflated or pushed in. This is because during the night temperatures most likely dropped. These cold temperatures during the night would have caused the air molecules inside the pool float to come closer together which would cause there to be more excess space inside the pool float. Therefore, allowing it to collapse into itself. This would be far worse if the float was filled with helium or another gas other than O2.
nonpathogenic or ""normal"" crystals found in acidic urine include:
Answers
Non-pathogenic or “normal” crystals found in acidic urine include uric acid crystals and calcium oxalate crystals.
Urine is a liquid excretory product that is produced by the kidneys, stored in the bladder, and expelled from the body during urination. It's made up of water and various waste products that your body eliminates as part of its normal metabolic processes.
Crystals in urine are tiny, solid deposits that form in the kidneys and bladder. These crystals can clump together and form stones. The size of these crystals varies from a few micrometers to a few millimeters.
Crystals that occur naturally in urine and are not linked to any medical problem are called non-pathogenic crystals. Uric acid crystals and calcium oxalate crystals are two examples of these. These crystals can appear in both healthy and unhealthy individuals. In acidic urine, uric acid crystals and calcium oxalate crystals are common. Urine pH is used to classify the urine as acidic, alkaline, or neutral.
You can learn more about acidic urine at: brainly.com/question/12993568
#SPJ11