Which pair of elements are the most common found in the sun?.
Answers
Hydrogen and helium are the most common elements found in the Sun. The Sun has an estimated composition of 70 percent hydrogen and 28 percent helium by mass, with heavier elements making up the remaining 2 percent.
Hydrogen and helium are the most prevalent elements in the Sun's composition. As stated before, hydrogen accounts for 70 percent of the Sun's mass, while helium accounts for 28 percent. The remaining 2 percent is composed of heavier elements such as carbon, oxygen, and iron.The Sun, like other stars, is a massive, glowing ball of plasma. The Sun's core is where hydrogen fusion takes place, producing helium as a byproduct. Helium is denser than hydrogen, so it gradually sinks towards the Sun's core, which causes the Sun's core to become denser over time. This increase in density raises the Sun's temperature and pressure, making it possible for hydrogen fusion to occur.The Sun's composition is critical in comprehending its properties and behavior. Because hydrogen fusion produces an enormous amount of energy, the Sun's composition allows it to shine brightly and provide warmth and light to Earth. Additionally, scientists utilize the Sun's composition as a guide for understanding the formation and evolution of the solar system.
To know more about Hydrogen visit :
https://brainly.com/question/31018544
#SPJ11
Related Questions
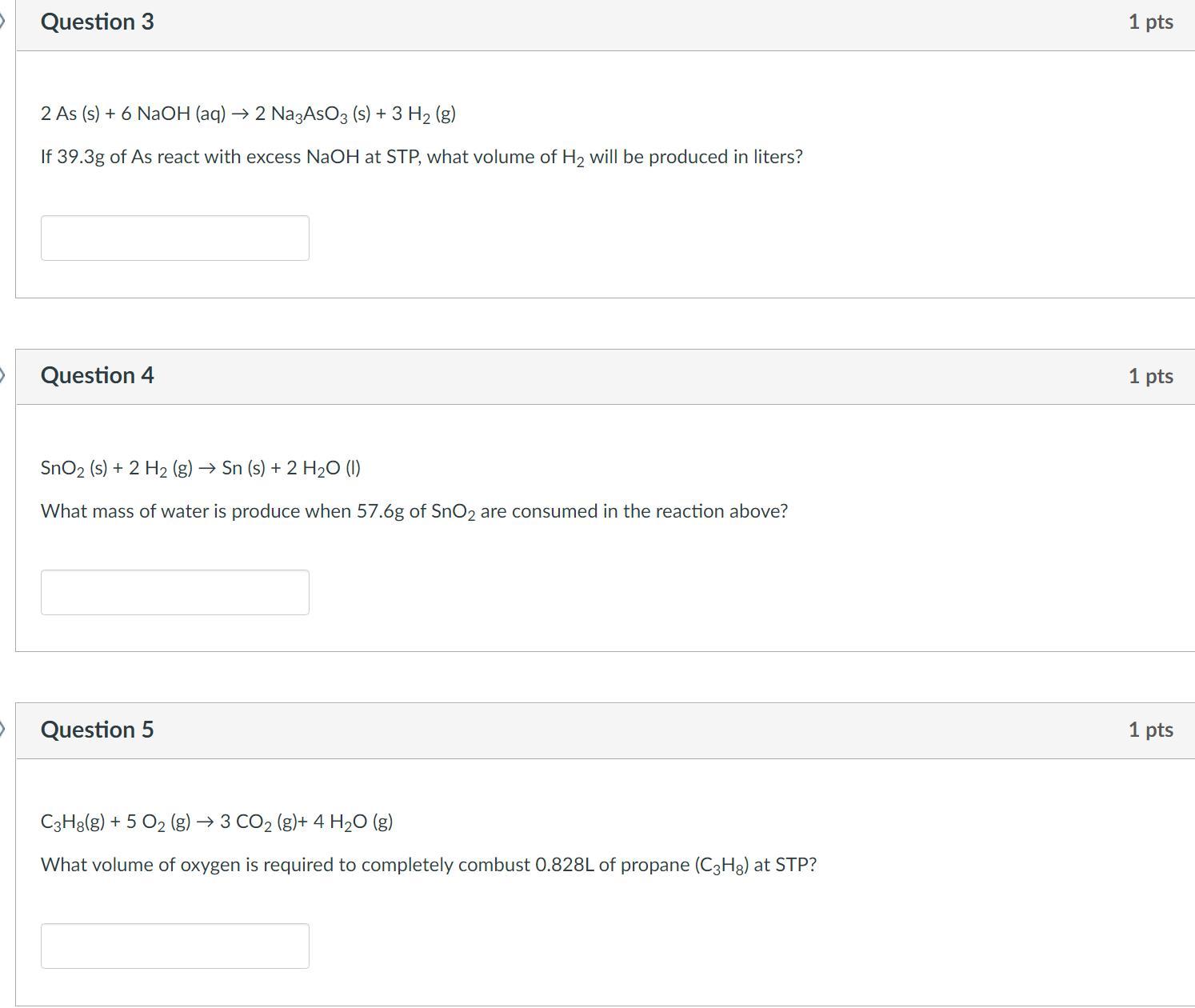
What volume of 0.200 M Na2CO3 (Mm = 106 g/mol) solution contains 53.0 g of Na2CO3?
Question 1 options:
0.200 L of solution
0.400 L of solution
0.500 L of solution
1.60 L of solution
2.50 L of solution
Answers
Answer:
volume in Liter = 2.50 L
Explanation:
Given:
Na2CO3 = 0.2 M
Molar mass of Na2CO3 = 106 g/mol
Mass of Na2CO3 solution = 53 gram
Find:
Volume of Na2CO3
Computation:
Number of mol of Na2CO3 = (53 g) / (1.06 x 10² g/mol)
Number of mol of Na2CO3 = 0.5 mol
M = Number of mol / volume in Liter
0.2 = 0.5/ volume in Liter
volume in Liter = 0.5 / 0.2
volume in Liter = 2.50 L
c-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. assume a rock starts with 1000 atoms of c-14. if the rock is 5730 years old, how many c-14 atoms should be left?
Answers
250 , C-14 atoms should be left, if the rock is 5730 years old. C-14 (radiocarbon) is radioactive and decays into n-14 with a half-life of 5730 years. assume a rock starts with 1000 atoms of C-14.
Radiation-emitting radiocarbon substances are referred to as radioactive . A radionuclide decays into a different atom known as a decay product. Until the atoms achieve a stable state and stop being radioactive, the radiocarbon continue to change into new decay products. C degrades through a process known as beta decay. One of the neutrons in the radiocarbon atom turns into a proton during this process, which results in the decay of a 14C atom into a 14N atom. By adding one more proton to the atom, this results in the formation of a nitrogen atom rather than a radiocarbon atom.
learn more about decay here:
https://brainly.com/question/9615302
#SPJ4
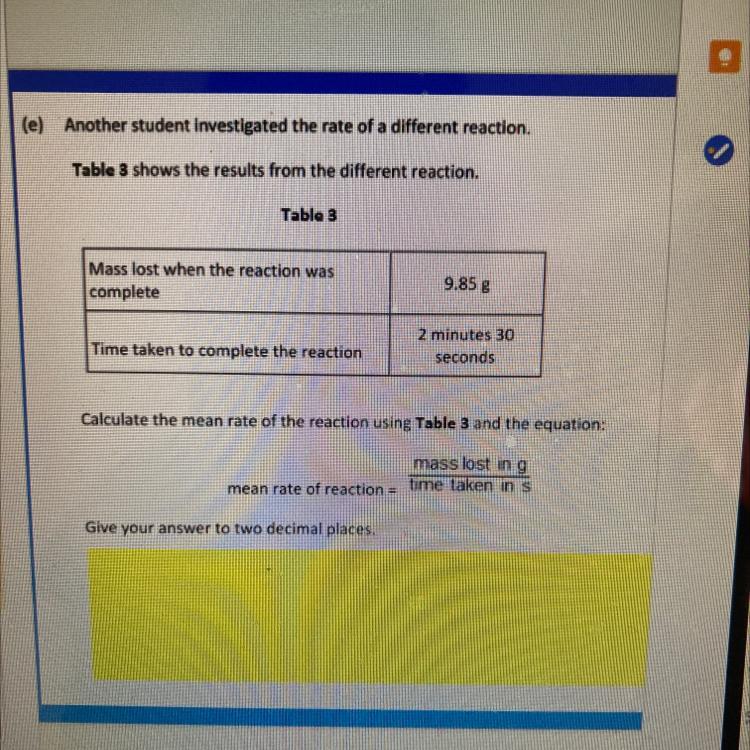
(e) Another student investigated the rate of a different reaction
Table 3 shows the results from the different reaction
Headings that you a
will appear here.
Table 3
Mass lost when the reaction was
complete
9.85 g
Time taken to complete the reaction
2 minutes 30
seconds
Calculate the mean rate of the reaction using Table 3 and the equation:
mass lost in g
mean rate of reaction time taken in s
Give your answer to two decimal places.
Answers
Answer:
0.07 g/s.
Explanation:
From the question given above, the following data were obtained:
Mass lost = 9.85 g
Time taken = 2 min 30 s
Mean rate =?
Next, we shall convert 2 min 30 s to seconds (s). This can be obtained as follow:
1 min = 60 s
Thus,
2 min = 2 × 60 = 120 s
Therefore,
2 min 30 s = 120 s + 30 s = 150 s
Finally, we shall determine the mean rate of the reaction. This can be obtained as illustrated below:
Mass lost = 9.85 g
Time taken = 150 s
Mean rate =?
Mean rate = mass lost / time taken
Mean rate = 9.85 / 150
Mean rate = 0.07 g/s
Therefore, the mean rate of the reaction is 0.07 g/s
Electrolytic capacitors differ from many other cpapacitors in construction in that they are?
Answers
the valency of sodium is one give reason
Answers
Answer:
here
Explanation:
The valency of sodium is 1 because it loses it's one valence electron to gain its octet state (state of having 8 electrons in shell) during the chemical reaction.
Please mark me as brainliest
Describe two ways to add heat to liquid water.
Answers
Answer:
There are several ways to add heat to liquid water. Some common methods include:
Using a heating element: This involves using a device such as a stovetop burner, electric heater, or immersion heater to transfer heat to the water. The heating element is typically in direct contact with the water, allowing heat to be transferred quickly and efficiently.
Using a heat exchanger: This involves using a device such as a radiator or heat pump to transfer heat from a hot fluid (such as steam or hot water) to the water. The heat exchanger typically has a separate flow of hot fluid on one side and the water on the other, with a metal or other material separating the two to allow heat to be transferred.
Using a phase change: This involves adding a substance to the water that can absorb heat as it changes phase, such as a solid that melts or a liquid that evaporates. For example, adding ice to water will cause the ice to melt, absorbing heat from the water in the process. Similarly, adding a volatile liquid such as alcohol to water will cause the alcohol to evaporate, absorbing heat from the water as it does so.
Please someone help I will give brainiest
Answers
From the balanced equation of the first reaction, the mole ratio of NaOH to \(H_2\) is 2:1.
Recall that: mole = mass/molar mass
Thus, 39.3 g of NaOH = 39.3/40 = 0.98 mol
The equivalent mole of \(H_2\) from the mole ratio = 09.8/2 = 0.49 mol
1 mol of gas at STP = 22.4 L
0.49 mol = 0.49 x 22.4 = 10.98 L of \(H_2\)
From the balanced equation of the second reaction, the mole ratio of \(SnO_2\) and water is 1:2.
57.6 g of \(SnO_2\) = 57.6/150.7 = 0.38 mol
Equivalent mole of water from the mole ratio = 0.38 x 2 = 0.76 mol
Since, mass = mole x molar mass
0.76 mol of water = 0.76 x 18 = 13.68 grams of water.
For the third equation, the mole ratio of propane and oxygen for a complete combustion reaction is 1:5. This can be converted to a volume ratio at STP.
Thus, with 0.828 L of propane, the equivalent volume of oxygen required for complete combustion would be:
0.828 x 5 = 4.14 L
More on stoichiometric problems can be found here: https://brainly.com/question/29856106
#SPJ1
how can you blance it and make it equal on both sides
2H2+o2=2H2o blance it
Answers
Answer:
it have been already balanced
2H2 + O2 = 2H2O.
What is the formula for Tetracarbon octahydtide?
Answers
Answer:
Tricarbon octahydride has the formula C 3 H 8.This means it has three carbon atoms and eight hydrogen atoms.
Explanation:
Answer:
C3 H8
uR wElCOme
you have a two-bulb system with a closed valve between the bulbs. the left bulb, 2.00 l, contains nitrogen gas with a pressure of 5.00 atm. the right bulb, 3.00 l, contains carbon dioxide gas with a pressure of 3.00 atm. you open the valve and let the gases mix. what is the mole fraction of carbon dioxide in the mixture?
Answers
The mole fraction of carbon dioxide is the ratio of its partial pressure to the total pressure. The mole fraction carbon dioxide in the mixture is 0.37.
What is mole fraction?Mole fraction of a gas in a mixture of gases is the ratio of its number of moles to the total number of moles. According to Dalton's law of partial pressure, the mole fraction of the gas in a mixture is equal to its fraction of partial pressure.
Given that, partial pressure of nitrogen = 5 atm
partial pressure of carbon dioxide = 3 atm
mole fraction = 3 atm / (3 + 5 atm) = 0.37.
Therefore, the mole fraction of carbon dioxide in the gaseous mixture is 0.37.
To find more on mole fraction, refer here:
https://brainly.com/question/8076655
#SPJ1
What are the base units the SI units are based on?
A-10
B-100
C-1,000
D-1
Answers
Answer:
A-10
Explanation:
In the SI, designations of multiples and subdivision of any unit may be arrived at by combining with the name of the unit the prefixes deka, hecto, and kilo meaning, respectively, 10, 100, and 1000, and deci, centi, and milli, meaning, respectively, one-tenth, one-hundredth, and one-thousandth.
IM NOT SURE PO
Which group contains the independent variable and dependent variable?
Answers
Answer:
Experimental group
Explanation:
This is a test sample or the group that receives an experimental procedure. This group is exposed to changes in the independent variable being tested. The values of the independent variable and the impact on the dependent variable are recorded.
Which process releases energy?
a. freezing
b. sublimation
c. condensation
d. evaporation
Answers
The process that releases energy is "condensation." Condensation is the process by which a gas or vapor changes phase into a liquid when it loses energy (heat).
As the gas molecules lose energy, they move closer together, and intermolecular forces cause them to form a liquid.
During condensation, energy is released into the surrounding environment in the form of heat. This energy is the same as the energy that was added to the substance during the process of evaporation, which is the opposite of condensation.
Freezing and sublimation are processes that require energy input, while evaporation is a process that involves energy absorption or input.
Learn more about condensation here brainly.com/question/956180
#SPJ4
Determine the carburizing time necessary to achieve a carbon concentration of 0. 30 wt% at a position 4 mm into an iron–carbon alloy that initially contains 0. 10 wt% C. The surface concentration is to be maintained at 0. 90 wt% C, and the treatment is to be conducted at 1100°C. Use the diffusion data for γ-Fe in Table 5. 2. ( Callister, Materials Science and Engineering, 9th ed. , John Wiley & Sons, Inc. , 2014) Express your answer in hours to three significant figures
Answers
The carburizing time necessary to achieve a carbon concentration of 0.30 wt% at a position 4 mm into an iron-carbon alloy is 63.4 hours.
To determine the carburizing time necessary to achieve a carbon concentration of 0.30 wt% at a position 4 mm into an iron-carbon alloy, we can use Fick's second law of diffusion:
\(DC_{surface} / 2 = (C_{surface} - C_{4mm}) / erf(x / (2 * \sqrt{Dt} ))\\\)
where D is the diffusion coefficient, \(C{surface}\\\) is the surface carbon concentration (0.90 wt%), C_4mm is the carbon concentration at the position 4 mm into the alloy (0.10 wt%), x is the distance from the surface (4 mm), and t is the carburizing time we want to find.
We can use the diffusion coefficient for γ-Fe at 1100°C from Table 5.2, which is D = \(6.0 * 10^{-12} m^2/s.\)
Substituting the given values, we get:
\((6.0 * 10^{-12} m^2/s) * (0.90 - 0.30) / 2 = (0.90 - 0.10) / erf(4 mm / (2 * \sqrt{6.0 * 10^{-12} m^2/s} ))\)
Simplifying the left-hand side, we get:
\(1.8 * 10^{-12} m^2/s = (0.80) / erf(4 mm / (2 * \sqrt{(6.0 * 10^{-12} m^2/s) * t)})))\)
Taking the inverse error function of both sides, we get:
\(erf(4 mm / (2 * \sqrt{6.0 * 10^{-12} m^2/s) * t)} ) = 0.000346\)
Substituting this back into the previous equation, we get:
\(1.8 * 10^{-12} m^2/s = (0.80) / 0.000346\)
Solving for t, we get:
t = 63.4 hours
For more question on diffusion click on
https://brainly.com/question/30900484
#SPJ11
To shape, form, or improve a metal through compression or stretching is to?
Answers
The process by which we pass a metal to shape, form, or improve a metal through compression or stretching is the process that we refers to as forging the metal.
What is metal forging?We know that metals can be used for a number of purposes. In fact the raw metal must be passed through a process that makes the metal quite fit for use. We refer to this process that the metal passes as the process of refining.
Now the process by which we pass a metal to shape, form, or improve a metal through compression or stretching is the process that we refers to as forging the metal.
Learn more about forging metals:https://brainly.com/question/11232019
#SPJ1
PLEASEEEE HELP DUE IN 2 HOURSS PLEASE!! 15 POINTS!!!!Someone decides to swap out nitric acid (HNO3) for hydrogen
chloride (HCI), given that it will be much stronger due to opposing dipole
forces. Explain if they are correct or incorrect and why.
*
Answers
Explanation:
The claim that hydrogen chloride (HCl) would be much stronger than nitric acid (HNO3) due to opposing dipole forces is incorrect.
Both HCl and HNO3 are strong acids, meaning that they dissociate completely in water to produce H+ ions. The strength of an acid is determined by the degree to which it dissociates in water. In other words, the stronger the acid, the more H+ ions it produces in water.
The dissociation of HCl and HNO3 in water can be represented as follows:
HCl + H2O → H+ + Cl-
HNO3 + H2O → H+ + NO3-
As we can see, both HCl and HNO3 produce H+ ions in water. Therefore, the strength of an acid cannot be solely determined by its dipole forces.
In addition, it's important to note that HCl is a much more volatile and corrosive acid than HNO3. It can cause severe respiratory and skin irritation when it is inhaled or comes into contact with skin. Therefore, switching HNO3 for HCl could be dangerous and should not be done without proper precautions and expert knowledge
Select all correct answers. Breaking the chemical bonds in reactions requires:
proper orientation of the molecules.
collisions between particles.
an overall release of energy.
sufficient kinetic energy to break the bonds.
an overall decrease in energy.
Answers
The correct answers are: proper orientation of the molecules; collisions between particles; and sufficient kinetic energy to break the bonds, as breaking chemical bonds in reactions requires proper orientation of the molecules and collisions between particles.
Chemical reactions involve the breaking of bonds between atoms in reactant molecues and the formation of new bonds between atoms in product molecules. Breaking these bonds requires a certain amount of energy, which can be supplied through collisions between particles and the proper orientation of the molecules involved in the reaction. When two reactant molecules collide, the orientation of their atoms is important. The atoms need to be in the correct position relative to each other for the chemical bonds to break and new bonds to form.
Learn more about chemical reactions and energy here.
https://brainly.com/question/11637586?
#SPJ1
Some weather conditions are shown.
Weather Conditions
• Cold front moving in behind warm, moist air
• Heavy, constant winds
• Sudden hail
• Unstable atmosphere
Which type of severe event is MOST likely to occur during these weather conditions?
Answers
Answer:
Tornado
Explanation:
This is because, tornados are heavy constant winds that are blowing around all over causing an unstable atmosphere. Sometimes the weather will change drastically like causing hail, and cold fronts moving in. This is exactly what matches the conditions you show! :)
Hope this helps! Please mark as brainliest!
water is to ____as liquid is to soild
Answers
water is to gas as liquid is to solid
Under what condition does the ideal gas line not apply and gases are considered real? Check all that apply
Answers
Answer:At high pressure and low temperature.
Explanation:
At high pressure voleme of a gas is'nt negligible as compared to the container
And at low temperature, kinetic energy of gas molecules lower, so they come closer to one another and intermolecular forces between them are considerable
What is the total volume of water that you have if you calculated that it
weighs 180g?
Answers
Which of the following describes the middle layers of the earth
Answers
What can be said about 1 mole of Ag and 1 mole of Au?

Answers
Explanation:
Avogadro's number. What can be said about 1 mol Ag and 1 mol Au? ... They contain the same number of atoms.
1 mole of Ag and 1 mole of Au contain the same number of particle. Therefore, option B is correct.
What is mole ?The International System of Units uses the mole (symbol: mol) as the unit of material quantity. How many elementary entities of a particular substance are present in an object or sample is determined by the quantity of that material. It is specified that the mole contains exactly 6.022140761023 elementary entities.
A mole is defined as the mass of a substance that has the same number of elementary particles as there are atoms in precisely 12.000 g of 12C.
In the periodic chart, group 11 includes the chemical elements roentgenium (Rg), copper (Cu), silver (Ag), and gold (Au). However, no chemical tests have yet been done to demonstrate that roentgenium behaves similarly to the heavier homologue of gold.
Thus, option B is correct.
To learn more about mole, follow the link;
https://brainly.com/question/26416088
#SPJ2
What is the pH of a solution containing 0.1M acetic acid and 0.1M sodium acetate? The pka of acetic acid is 4.76. Record your answer to two decimal places.
Answers
The pH of the solution is 4.76, which means it is slightly acidic. The pH of the solution containing 0.1M acetic acid and 0.1M sodium acetate can be calculated using the Henderson-Hasselbalch equation, which is pH = pKa + log([A-]/[HA]).
In this case, the acetic acid is the weak acid (HA) and its conjugate base, sodium acetate, is the weak base (A-). Therefore, pKa = 4.76, [A-] = 0.1 M, and [HA] = 0.1 M. Substituting these values into the equation, we get pH = 4.76 + log(0.1/0.1) = 4.76 + 0 = 4.76.
In this case, pKa = 4.76, [A-] represents the concentration of sodium acetate (0.1M), and [HA] represents the concentration of acetic acid (0.1M). Substituting these values, we have pH = 4.76 + log(0.1/0.1). Since log(1) = 0, the pH = 4.76 + 0.
Therefore, the pH of the solution is 4.76.
To know about acidic :
https://brainly.com/question/29796621
#SPJ11
How many grams of h3po3 would be produced from the complete reaction of 93.2 g p2o3
Answers
Answer: 137.76 g
Explanation:
during a nuclear reaction, what is mass converted into?
Answers
Answer:
The mass is converted to energy to cause the mushroom cloud you woud see
Explanation:
A horticulturalist wants to produce geraniums with specific characteristics.
She knows that the trait of red flowers is governed by the allele R (RR and Rr) and the trait of white flowers is governed by the allele r(rr).
The horticulturalist crosses two geraniums from the F1 generation.
Look at the Punnett square to see this cross.
R r
R RR Rr
r Rr rr
Which ratio of red-to-white flowering plants would she expect to see in the phenotypes of the F2 generation?
all red
3 red : 1 white
1 red : 3 white
2 red : 2 white
Answers
Yo,
In Geranium plant life red vegetation is given by using the allele R this is dominant (so RR or Rr ) genotype will produce crimson plant life.
If we communicate approximately the connected punnet square which i took from the workout e book, you could see that there was a cross made among vegetation with heterozygous genotype for red flora.
P1: Rr X Rr
Gametes: R: r : R : r
F1: RR: Rr: Rr: rr
so there is three: 1 in F1 offspring for red to white plant life.
Now the query is set f2 technology, while we cross F1 generation suppose we do a move of Rr and rr from F1 generations allow us to see what takes place.
F1: Rr: rr
Gametes: R: r: r: R
F2 : Rr : rr: Rr: rr
Rr: purple flowers
rr: White flora:
So the ratio in F2 era might be 2 crimson : 2 white. Please see connected punnet squares (first one for query) and the second of F1 move for better know-how.
I'm hoping that this will benefit you, so from my assumption and study it'd be 2 crimson : 2 white, if this is indeed the one i'm thinking of since nothing else was provided, if not please correct me.

Answer: 2 red : 2 white
Explanation:
I know
Mr. Wang works in a recycling center. Recyclable materials arrive at the center mixed. Workers use magnets to separate steel cans from other items. Which two statements are true about the force between a steel can and a magnet?
Answers
Answer:
Option 3, The attraction between the can and the magnet is a pull.
Explanation:
The complete question is
Mr. Wang works in a recycling center. Recyclable materials arrive at the
center mixed together. Workers use magnets to separate steel cans from
other items. Which two statements are true about the force between a steel can and a magnet?
1 Gravity pushes the can toward the magnet.
2 The force between the can and the magnet is a noncontact force.
3 The attraction between the can and the magnet is a pull.
4 The attraction between the can and the magnet is a push
Solution
The force exerted by magnet on steel is the pull force. In magnets unlike poles attract each other (pull force) while the like poles repel (push force). Now, the steel or any ferrous object in the garbage when experience magnetic field develop magnetic field of their own in such a way that their north always faces the south of the external magnet or vice versa.
Hence, the force between a steel can and a magnet is pull force
which formulas represent compounds that are isomers of each other?

Answers
Answer:
Option D.
Explanation:
Isomerism is a phenomenon where by two or more compounds have the same molecular formula but different structural patterns. The compounds involved are called isomers.
Option A has two different compound with two different molecular formula. Hence they are not isomers.
Option B has two different compound with two different molecular formula. Hence they are not isomers
Option C can not be called isomers because Isomerism can not occur in compound having just 1 carbon atom.
Option D has two different compound with the same molecular formula as C3H8O and their structure are different. Hence they areisomers.
Calculate the mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution.
Answers
The mass of (NH4) 2S in the solution is : Mass = 0.0600 mol × 60.08 g/mol = 3.60 g.
The given molarity and volume of the solution can be used to calculate the number of moles of ammonium sulfide (NH4)2S.Then, the number of moles can be converted to mass using the molar mass of (NH4)2S.Mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution is given by : Mass = moles × molar mass.The number of moles of (NH4)2S can be found using the equation:Molarity = Number of moles / Volume.Rearranging this equation, we get:Number of moles = Molarity × Volume Number of moles of (NH4)2S = 0.0200 M × 3.00 L.Number of moles of (NH4)2S = 0.0600 mol.The molar mass of (NH4)2S can be calculated by summing the molar masses of ammonium (NH4) and sulfide (S) ions.Molar mass of (NH4)2S = (2 × Molar mass of NH4) + Molar mass of S= (2 × 14.01 g/mol) + 32.06 g/mol= 60.08 g/mol.
For more question on mass
https://brainly.com/question/1838164
#SPJ8