Which reactions performed in the experiment
involved chemical changes?
crushing calcium carbonate
mixing calcium carbonate and HCI
boiling water
heating copper (II) sulfate pentahydrate
separating iron filing and sulfur
mixing potassium iodide and lead nitrate
combining magnesium and HCI
burning the candle
DONE
Answers
Mixing calcium carbonate and HCl
Heating copper sulfate pentahydrate
Mixing potassium iodide and lead nitrate
Combining magnesium and HCl
Burning the candle.
The crushing of limestone is a bodily trade it no longer alters the chemical composition of the limestone. The heating of limestone is a chemical trade the limestone decomposes into two other materials, lime and carbon dioxide. both ended in gas formation. both ended in shade change.
Rust is not anything however Iron Oxide is a brand new substance shaped out of the response. The color of the floor of the iron additionally modifications. hence, rusting of iron is a chemical trade. Rusting is an instance of chemical exchange. A chemical property describes the ability of a substance to go through a particular chemical alternate. The chemical belonging of iron is that it is miles capable of combining with oxygen to form iron oxide, the chemical call of rust. like several steel carbonates, calcium carbonate reacts with acidic solutions to supply carbon dioxide gas.
Learn more about Experiment here:-https://brainly.com/question/17274244
#SPJ9
Related Questions
superficial zone chondrocytes can get compacted under physiological loading: a multiscale finite element analysis.
a. true
b. false
Answers
The statement "Superficial zone chondrocytes can get compacted under physiological loading: a multiscale finite element analysis" is true.
Superficial zone chondrocytes are the most exposed to mechanical loading and are therefore at risk of being compacted under physiological loading. A multiscale finite element analysis can be used to model and predict the mechanical behavior of cartilage under physiological loading, allowing for a better understanding of the factors that affect its mechanical properties.
This means that chondrocytes in the superficial zone of cartilage are at risk of being compacted under the physiological loading that it experiences in everyday life. A multiscale finite element analysis can be used to model and predict the mechanical behavior of cartilage under physiological loading, allowing for a better understanding of the factors that affect its mechanical properties.
Learn more about chondrocytes
https://brainly.com/question/28231704
#SPJ4
What is the heat, q, in calories transferred by a chemical reaction to the reservoir of a calorimeter containing 125 g of dilute aqueous solution (c=1.00calg∘C) if the reaction causes the temperature of the reservoir to rise from 21.5∘C to 24.5∘C?
Answers
The reservoir receives q = (125 g) x (1.00 cal/g C) x (3.0 C) = 375 cal of heat transfer.
With a calorimeter, how do you determine the heat of reaction?Equation q = -CT, where C is the calorimeter's heat capacity and T is the temperature change, can be used to determine how much heat is released during the reaction. As the combustion takes place at a constant volume, the reaction's q is equal to E.
A calorimeter's heat transfer, q, to the reservoir can be computed using the following formula:
q = mcΔT
Inputting the values provided yields:
m = 125 g
c = 1.00 cal/g∘C
ΔT = 24.5∘C - 21.5∘C = 3.0∘C
As a result, the reservoir received the following heat transfer:
q = (125 g) x (1.00 cal/g∘C) x (3.0∘C) = 375 cal
The heat released by the reaction is q = -375 cal as a result of the heat being transported to the reservoir.
To know more about reservoir visit:-
https://brainly.com/question/14142895
#SPJ1
plz help ill give u brainiest
Answers
Answer:
Supercalifragilisticexpialidocious...
Explanation: beacause...so...yeah...
Which transformation will create $Q'R'S'T'$ , a quadrilateral that is similar but not congruent to quadrilateral $QRST$ ?
Answers
To create a quadrilateral that is similar but not congruent to another quadrilateral, you can apply a non-uniform dilation transformation.
What is non-uniform dilation transformation?A non-uniform dilation transformation changes the size of a shape by a different scale factor in different directions. This means that the corresponding sides of the similar figures will have different lengths, and the corresponding angles will have the same measure.
To apply a non-uniform dilation transformation to quadrilateral $QRST$, you would need to specify different scale factors for each direction. For example, you could specify a scale factor of 2 in the x-direction and a scale factor of 3 in the y-direction. The resulting transformed quadrilateral, $Q'R'S'T'$, would have sides that are twice as long as the corresponding sides in the x-direction and three times as long as the corresponding sides in the y-direction. The angles of $Q'R'S'T'$ would be the same as the angles of $QRST$.
It is important to note that a non-uniform dilation transformation will not preserve the ratios of side lengths between similar figures, so $Q'R'S'T'$ will not be congruent to $QRST$.
Learn more about non-uniform dilation transformation, here:
https://brainly.com/question/18242733
#SPJ9
What is the molarity (M) of a bleach solution containing 9.50 grams of bleach (NaOCI) in 2,000 ml of solution?
Answers
The molarity of the bleach solution is 0.0635 M.
How to calculate the molarity (M) of a solution ?First we need to know the number of moles of solute (in this case, NaOCI) and the volume of the solution in liters.
First, we need to convert the mass of NaOCI to moles using its molar mass:
molar mass of NaOCI = 23.0 g/mol (for Na) + 16.0 g/mol (for O) + 35.5 g/mol (for Cl) + 1.0 g/mol (for I) = 74.5 g/mol
moles of NaOCI = mass / molar mass = 9.50 g / 74.5 g/mol = 0.127 moles
Next, we need to convert the volume of the solution to liters:
volume = 2,000 ml = 2,000 / 1,000 L = 2.0 L
Now we can calculate the molarity of the solution:
Molarity (M) = moles of solute / volume of solution in liters
M = 0.127 moles / 2.0 L = 0.0635 M
Therefore, the molarity of the bleach solution is 0.0635 M.
Learn more about molarity here : brainly.com/question/17138838
#SPJ1
a tire will burst if the air inside it reaches a pressure greater than 1.4 x 10^3 kpa. at what temperature will the tire burst if it has a volume of 30L and contains 2.5 mol of air? assume that the air behaves as an ideal gas. assuming that these values are representative, do you need to worry about your car tire bursting from overheating of they are in good condition?
Answers
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
To determine the temperature at which the tire will burst, we can use the ideal gas law equation:
PV = nRT
Where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Rearranging the equation to solve for temperature, we have:
T = PV / (nR)
Given that the pressure threshold for bursting is 1.4 x 10^3 kPa, the volume is 30 L, and the number of moles of air is 2.5 mol, we can substitute these values along with the ideal gas constant R = 8.314 J/(mol K) into the equation.
T = (1.4 x 10^3 kPa) * (30 L) / (2.5 mol * 8.314 J/(mol K))
Converting kPa to Pa and L to m^3, and simplifying the equation, we find:
T ≈ 20,993 K
This extremely high temperature indicates that under normal conditions, you do not need to worry about your car tire bursting from overheating as it is unlikely to reach such extreme temperatures.
For more question on temperatures
https://brainly.com/question/4735135
#SPJ8
A buffer solution is prepared by dissolving 0.2 mol of CH3NH2 (KB=3.7×10−4) and 0.1 mol of CH3NH3Cl in enough water to make 1.00 L of solution. Determine the pH of the buffer
Answers
The pH of acid is between 0-7 on pH scale while for base pH range is from 7-14. Therefore, the pH of buffer is 10.5. pH is a unitless quantity.
What is pH?pH is a measurement of amount of hydronium ion H₃O⁺ in a given sample. More the value of hydronium ion concentration, more will be the solution acidic.
On subtracting pH from 14, we get pOH which measures the concentration of hydroxide ion in a given solution. pH depend on the temperature. At room temperature pH scale is between 0 to 14. pH of neutral solution is 7.
CH\(_3\)NH\(_2\) + H\(_2\)O(aq) \(\rightarrow\) CH\(_3\)NH\(_3\) ⁺(aq) + OH⁻(aq)
Kb = [CH\(_3\)NH\(_3\) ⁺] x[OH⁻] =3.7x 10⁻⁴
[CH\(_3\)NH\(_2\)]= 0.2 / 1.00 =0.2M
Kb = (0.2M) x [OH⁻] = 3.7x 10⁻⁴
[OH-] = 3.15 x 10⁻⁴
pOH=-log[OH-]
= -log[3.15 x 10⁻⁴]
pOH=3.5
pH+pOH=14
pH = 10.5
Therefore, the pH of buffer is 10.5.
To learn more about pH, here:
https://brainly.com/question/27945512
#SPJ1
The weather on Earth is influenced by the oceans, which store, release, and redistribute energy through evaporation. The source of this energy is–
A. solar radiation
B. gravitational attraction
C. density differences
D.chemical reactions
Answers
I am just typing to keep up space cuz I need 20 spaces
1. Someone Please help me! List the main types of EMR that have an impact on living tissue. Explain this impact and provide examples from this lesson.
2. Eating one banana is equivalent to receiving 0.01 mrem. How many bananas would you have to consume before you risk increasing your probability of developing cancer? Explain your answer.
Answers
2. Eating one banana is equivalent to receiving 0.01 mrem of radiation. The average person in the US receives about 300 mrem of radiation per year from natural sources, such as cosmic rays and radon gas. The risk of developing cancer from radiation exposure depends on the dose received, with higher doses increasing the risk. According to the National Cancer Institute, the risk of developing cancer from radiation exposure is about 5% per 1000 mrem of exposure. This means that eating 33,000 bananas (33,000 x 0.01 mrem = 330 mrem) would increase the risk of developing cancer by about 5%. However, this is a very high number of bananas, and it is unlikely that anyone would eat this many bananas in a short period of time. In general, the risk of developing cancer from eating bananas or other natural sources of radiation is very low compared to other sources of radiation exposure, such as medical imaging or nuclear accidents.
2. How many grams are in 2.05 x 1030 atoms of Lithium (Li)?
Answers
The drawing shows Earth's revolution around the Sun.
Based on the drawing, what season are students in Florida experiencing when Earth is at position 1?
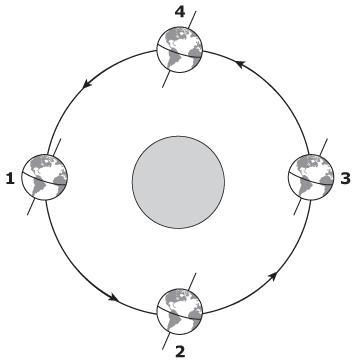
Answers
According to the image, it can be inferred that the students in Florida are in summer when the earth is in position 1.
What relationship does the movement of the earth have with the seasons?The seasons are a term that refers to each of the periods of time in which the year is divided, with a duration greater than the months, characterized by the typical behavior of some meteorological variable. They occur cyclically and inverted between one hemisphere and another.
In general, the seasons are divided in this way:
Position 1: June 21 - September 23
Boreal Summer
I invest Austral
Position 2: September 23 - December 21
boreal autumn
southern spring
Position 3: December 21 - March 21
boreal winter
Ausral Summer
Position 4: March 21 - June 21
boreal spring
southern autumn
According to the above, it can be inferred that in position 1 the students from Florida will be in the summer season because Florida is in the boreal hemisphere of the earth (Northern hemisphere).
Learn more about seasons of the year in: https://brainly.com/question/11018528
#SPJ1
copper exists as a mixture of 2 isotopes, 70.5% Cu-63, with a mass of 62.96 amu, and 29.5% Cu-65, with a mass of 64.96 amu. what is the average atomic weight?
Answers
Relative and average atomic mass both describe properties of an element related to its different isotopes. Out of these two Relative atomic mas is more accurate. Therefore, the average atomic mass of copper is 63.55amu.
What is mass?Mass defines the quantity of a substance. It is measured in gram or kilogram. Average mass is the mass of atoms of an element that are isotopes. It can be calculated by multiplying mass of a isotope to natural abundance of that isotope.
Average atomic mass = (mass of first isotope× percent abundance of first isotope)+(mass of second isotope× percent abundance of second isotope)
Substituting the given values, we get
Average atomic mass=( 62.96 amu × 70.5% )+(64.96 amu ×29.5% )
Average atomic mass=55.58+12.27
Average atomic mass=63.55amu
Therefore, the average atomic mass of copper is 63.55amu.
To learn more about mass, here:
brainly.com/question/28704035
#SPJ1
Think of an example in which chemical and physical weathering affects a rock
Answers
Answer:
chemical-Acid rain eating away at the rock
Physical-Dust particles eroding the rock overtime in the wind
Explanation:
Which of these four elements is the most reactive metal?
Answers
Answer:
Rubidium
Answer: Rubidium is the most reactive metal. Explanation: Metals are the elements that looses electrons and thus, their chemical reactivity will be the tendency to loose electrons.
Explanation:
What is the theoretical yield of BOTH products when 100.0 g of Cr2O3 reacts with 100.0 g of C?
Answers
Theoretical yield of Cr is 68.43 g and the theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
What is theoretical yield?Quantity of a product obtained from complete conversion of the limiting reactant in chemical reaction is called theoretical yield.
2 Cr2O3 + 3 C → 4 Cr + 3 CO2
Molar mass of Cr2O3 is 152 g/mol, and the molar mass of C is 12.01 g/mol. Therefore, 100.0 g of each reactant is equivalent to:
100.0 g Cr2O3 / 152 g/mol = 0.6579 mol Cr2O3
100.0 g C / 12.01 g/mol = 8.329 mol C
The stoichiometry of the balanced equation tells us that for every 2 moles of Cr2O3, we need 3 moles of C. Therefore, if we have 0.6579 mol of Cr2O3, we need (3/2) x 0.6579 = 0.9868 mol of C to react completely. However, we only have 8.329 mol of C, which is in excess. This means that Cr2O3 is limiting reactant, and C is excess reactant.
From the balanced equation, 2 moles of Cr2O3 produce 4 moles of Cr.
Therefore, 0.6579 mol of Cr2O3 will produce (4/2) x 0.6579 = 1.316 mol of Cr.
Molar mass of Cr is 52.0 g/mol, so the theoretical yield of Cr is 1.316 mol x 52.0 g/mol = 68.43 g.
Therefore, 8.329 mol of C will produce (3/1) x 8.329 = 24.99 mol of CO2.
Molar mass of CO2 is 44.01 g/mol, so the theoretical yield of CO2 is 24.99 mol x 44.01 g/mol = 1099.25 g or 1.1 kg.
Therefore, theoretical yield of Cr is 68.43 g and theoretical yield of CO2 is 1.1 kg when 100.0 g of Cr2O3 reacts with 100.0 g of C.
To know more about theoretical yield, refer
https://brainly.com/question/25996347
#SPJ1
From the following heats of combustion,
CH₂OH(1) + 2O₂(g) → CO₂(g) + 2H₂O(1)
AHxn
= -726.4 kJ/mol
C(graphite) + O₂(g)
- CO₂(g)
→
AHxn
= -393.5 kJ/mol
H₂(g) + O₂(g) →→→ H₂O(1)
AHxn=-285.8 kJ/mol
calculate the enthalpy of formation of methanol
(CH3OH) from its elements:
C(graphite) + 2H₂(g) + O₂(g) → CH₂OH(1)
Answers
Answer:
To calculate the enthalpy of the formation of methanol (CH3OH) from its elements, you can use the following equation:
ΔHf(CH3OH) = [ΔHf(CO2) + 2ΔHf(H2O)] - [ΔHf(C) + 2ΔHf(H2) + ΔHf(O2)]
where ΔHf represents the enthalpy of formation.
Using the enthalpies of combustion provided in the question, you can calculate the enthalpy of the formation of methanol as follows:
Explanation:
ΔHf(CH3OH) = [ΔHf(CO2) + 2ΔHf(H2O)] - [ΔHf(C) + 2ΔHf(H2) + ΔHf(O2)]
= [-393.5 kJ/mol + 2(-285.8 kJ/mol)] - [-726.4 kJ/mol + 2(-285.8 kJ/mol) + 0]
= -393.5 kJ/mol + 2(-285.8 kJ/mol) - (-726.4 kJ/mol + 2(-285.8 kJ/mol) + 0)
= -393.5 kJ/mol + (-285.8 kJ/mol - 285.8 kJ/mol) - (-726.4 kJ/mol + (-285.8 kJ/mol - 285.8 kJ/mol) + 0)
= -393.5 kJ/mol - (-726.4 kJ/mol + 0)
= -393.5 kJ/mol + 726.4 kJ/mol
= 332.9 kJ/mol
Therefore, the enthalpy of the formation of methanol is 332.9 kJ/mol.
It's important to note that this calculation is based on the assumption that the enthalpies of combustion provided in the question are standard enthalpies of combustion, which are defined as the enthalpy changes that occur when one mole of a substance is burned in excess oxygen at standard temperature and pressure (STP). If the enthalpies of combustion are not standard enthalpies, the calculated enthalpy of the formation may not be accurate.
To learn more about Enthalpy formation
https://brainly.com/question/1621852
cyanate ion waste solution from gold-mining operations can be destroyed by treatment with hypochlorite ion in basic solution. Write a balanced oxidation-reduction equation for this reaction. OCN^-(aq) +OCl^-(aq) --> CO2^-(aq)+N2(g)+Cl^-(aq)+H2O(l)
Answers
The balanced oxidation-reduction equation for the destruction of cyanate ion waste solution from gold-mining operations by treatment with hypochlorite ion in basic solution is:
OCN⁻(aq) + OCl⁻(aq) + 2OH⁻(aq) → CO₂⁻(aq) + N₂(g) + Cl⁻(aq) + H₂O(l)
In this reaction, the cyanate ion (OCN⁻) is oxidized to carbon dioxide (CO₂⁻) and nitrogen gas (N₂), while the hypochlorite ion (OCl⁻) is reduced to chloride ion (Cl⁻). The reaction takes place in basic solution, which provides the hydroxide ions (OH⁻) needed to neutralize the acidic H⁺ ions produced during the oxidation of the cyanate ion.
The reaction is exothermic, releasing heat energy as the products form. This reaction is an effective way to dispose of the cyanate ion waste generated by gold-mining operations, as it converts the hazardous waste into harmless gases and ions.
To learn more about oxidation-reduction equation, here
https://brainly.com/question/13699873
#SPJ1
Circle the functional groups and I need help naming 7) and 10)

Answers
Answer:
The answer to your question is given below.
Explanation:
1. The functional group is alkanol or alcohol (R–OH).
2. The functional group is alkanone or ketone (RC=OR).
3. The functional group is alkanal or aldehyde (R–CHO)
4. The functional group is alkanoic or carboxylic acidic (R–COOH).
5. The functional group is ether (ROR)
6. The functional group is ester (RCOOR).
7. The functional group is alkanol or alcohol (R–OH).
8. The functional group is alkanoic or carboxylic acidic (R–COOH).
9. The functional group is alkanal or aldehyde (R–CHO).
10. The functional group is alkanone or ketone (RC=OR).
Naming of the compound
To name the above compound, we must do the following:
1. Locate the longest continuous carbon chain. This gives the parent name.
2. Identify the functional group.
3. Identify and locate the position of the substituent group attached if there are any.
4. Combine the above to obtain name of the compound.
Now, let us name compound 7 and 10.
7a. The longest carbon chain is 4 i.e butane.
b. The functional group is –OH i.e alkanol or alcohol. We'll replace the –e in butane with –ol to obtain the name.
Therefore, the name of the compound is butanol.
10a. The longest carbon chain is 4 i.e butane.
b. The functional group is RC=OR i.e alkanone or ketone. We'll replace the –e in butane with –one to obtain the name.
Therefore, the name of the compound is butanone.
According to Le Châtelier’s principle, does the equilibrium
shift in the direction of products or reactants when N2
is
added to the equilibrium mixture of each of the following
reactions? 110.52
a. 2NH31g2 hv 3H21g2 + N21g2
b. N21g2 + O21g2 hv 2NO1g2
c. 2NO21g2 hv N21g2 + 2O21g2
d. 4NH31g2 + 3O21g2 hv 2N21g2 + 6H2O1g2
Answers
According to Le Châtelier’s principle or reactions in chemical equilibrium;
2 NH₃ (g) → 3 H₂ (g) + N₂(g) : addition of N₂ shifts equilibrium to the left
N₂ (g) + O₂(g) → 2 NO (g) : addition of N₂ shifts equilibrium to the right
2 NO (g) → N₂ (g) + O₂ : addition of N₂ shifts equilibrium to the left
4 NH₃ (g) + 3 O₂ (g) → 2 N₂ (g) + 6 H₂O (g) : addition of N₂ shifts equilibrium to the left
What is equilibrium in a chemical reaction?Equilibrium in a chemical reaction refers to the point in a chemical reaction in which the rate of the formation of product from the reactants or the forward reaction is equal to the rate of formation of reactants from products or the backward reaction.
For equilibrium to be achieved in a chemical reaction the reaction must occur in a closed or isolated system.
The condition that occur in a reaction in equilibrium are described by Le Châtelier’s principle which states that for a reaction in equilibrium, the equilibrium position will shift to annul any constraint or change imposed on the system.
Considering the given reactions:
2 NH₃ (g) → 3 H₂ (g) + N₂(g) : addition of N₂ shifts equilibrium to the left
N₂ (g) + O₂(g) → 2 NO (g) : addition of N₂ shifts equilibrium to the right
2 NO (g) → N₂ (g) + O₂ : addition of N₂ shifts equilibrium to the left
4 NH₃ (g) + 3 O₂ (g) → 2 N₂ (g) + 6 H₂O (g) : addition of N₂ shifts equilibrium to the left
Learn more about chemical equilibrium at: https://brainly.com/question/5081082
#SPJ1
Step 5: Measure the Mass of the Water (Iron)
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g.
g
DONE✔
ZERO

Answers
Answer:
12.42 g
Explanation:
because I saw your laptop so it is
Different atoms and elementary particles have varied masses. Therefore, 12.42 g is the Mass of the Water.
What is mass?A body's mass is an inherent quality. Before the invention of the atom or particle physics, it was widely considered to be tied to the amount of matter inside a physical body. It was discovered that, despite having the same quantity of matter in theory, different atoms and elementary particles have varied masses.
There are various conceptions of mass in contemporary physics that are theoretically different but physically equivalent. The resistance of the body to acceleration in the presence of a net force can be measured experimentally as mass. 12.42 g is the Mass of the Water.
Therefore, 12.42 g is the Mass of the Water.
To know more about mass, here:
https://brainly.com/question/28704035
#SPJ5
47. If the molar mass of X(HCO3)2 is 162 g mol-¹, determine the relative atomic mass of X. [H=1.0, C = 12.0. O = 16.0] A. B. C. D. 40 48 61 101 please answer with step by step solution
Answers
Answer:
X(HCO3)2 is X2(C2H2O6), so the total number of atoms for each element can be calculated:
2 X atoms
4 H atoms
6 C atoms
12 O atoms
Calculate the total atomic mass of the elements:
2 X atoms * atomic mass of X = 2X * atomic mass of X
4 H atoms * 1.0 amu = 4.0 amu
6 C atoms * 12.0 amu = 72.0 amu
12 O atoms * 16.0 amu = 192.0 amu
The molar mass of X(HCO3)2 is 162 g/mol, so the total atomic mass of the elements should add up to 162 g/mol:
2X * atomic mass of X + 4.0 amu + 72.0 amu + 192.0 amu = 162 g/mol
Solve for X by rearranging the equation:
2X * atomic mass of X = 162 g/mol - 4.0 amu - 72.0 amu - 192.0 amu = -12.0 amu
2X = -12.0 amu / atomic mass of X
Divide both sides by 2:
X = -6.0 amu / atomic mass of X
So the relative atomic mass of X is 6.0 amu.
A chemist must prepare of nitric acid solution with a pH of at . He will do this in three steps: Fill a volumetric flask about halfway with distilled water. Measure out a small volume of concentrated () stock nitric acid solution and add it to the flask. Fill the flask to the mark with distilled water. Calculate the volume of concentrated nitric acid that the chemist must measure out in the second step. Round your answer to significant digits.
Answers
Answer:
The volume of concentrated nitric acid is
4.3mL
Explanation
Check the attachment

Which of the following best defines molar mass?
A. the mass of a mole of moles
B. the mass of one molecule of a substance
C. the mass of one atom of a substance
D. the mass of one mole of a substance
Answers
Answer:
D. the mass of one mole of a substance
Explanation:
The molar mass of a substance is the mass in grams of one mole of the substance.
For an element, the molar mass is the relative atoms mass expressed in grams. For example 23g of Na, 40g of oxygenFor compounds, molar mass is the gram -formula or gram - molecular weight. This is determined by the addition of its component atomic masses and then expressed in grams.18 gm glucose is dissolved in 90 gm of water. The relative lowering of vapour pressure is equal to
Answers
Answer:
0.02
Explanation:
Molecular mass of water= 2×1+1×16=18g
=2×1+1×16=18g
For 90g178.2g water nₐ =5
Molecular mass of glucose 6×12+12×1+6×16=180g
For 18g glucose nb =0.1
Now, X B = 0.1 / 0.1 + 5=0.0196 ~0.02
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
The phenomenon that causes air masses and water to bend or curve is the ______________.
PLZ I NEED HELP ILL GIVE BRAINLIEST
Answers
Answer:
The Coriolis effect
Explanation:
The Coriolis effect is the effect that makes tornados, water spouts, and are often seen in storms. They make water curve and rotate as well as the wind And current.
what's the most lightest element known?
Answers
Answer:
Hydrogen
Explanation:
Hydrogen, most abundant in the universe, is the chemical element with atomic number 1, and an atomic mass of 1.00794 amu, the lightest of all known elements. It exists as a diatomic gas (H2).
Answer:
Hydrogen
Explanation:
Hydrogen, most abundant in the universe, is the chemical element with atomic number 1, and an atomic mass of 1.00794 amu, the lightest of all known elements. It exists as a diatomic gas (H2).
Calculate the mass of calcium carbonate that must be decomposed to produce 25.0 L of carbon dioxide gas at 26 °C and 0.997 atm . How many liters of CO2 would be produced if there was only a 78% yield?
Answers
The mass of calcium carbonate that must be decomposed to produce 25.0 L of carbon dioxide gas at 26 °C and 0.997 atm is 104.1 g
The volume of CO₂ produced with a 78% yield would be 19.7 L.
What mass of calcium carbonate must be decomposed to produce 25.0 L of carbon dioxide gas at 26 °C and 0.997 atm?The mass of calcium carbonate that must be decomposed to produce 25.0 L of carbon dioxide gas at 26 °C and 0.997 atm is calculated from the equation of the reaction as follows:
The balanced equation of reaction: CaCO₃(s) → CaO(s) + CO₂(g)
The number of moles of CO₂ produced is determined from the ideal gas equation:
n = PV/RT
n = (0.997 atm) × (25.0 L) / [(0.0821 L·atm/(mol·K)) × (299 K)]
n = 1.04 mol CO₃
From the balanced equation;
1 mol of CaCO₃ produces 1 mol of CO₂.
Moles of CaCO₃₃required = 1.04 mol of CaCO3.
the mass of CaCO3 needed will be:
mass = 1.04 mol × 100.09 g/mol
mass = 104.1 g
If there is only a 78% yield, the actual moles of CO₂ produced would be:
moles = 0.78 × 1.04 mol
moles = 0.81 mol
solving for volume using the ideal gas equation:
V = nRT/P = (0.81 mol) × (0.0821 L·atm/(mol·K)) × (299 K) / (0.997 atm)
V = 19.7 L
Learn more about calcium carbonate at: https://brainly.com/question/29369494
#SPJ1
Predict the products in the chemical reaction, Na+AlN
Answers
NEED ASAP!
What is the resolution of a monochromator, Δλeff, with a exit slit width of 500 micrometers and a \(D^{-1}\) of 1.8 nm/mm? Express the answer in nm.
Answers
The resolution of the monochromator is 3.6nm
What is a MonochromatorA monochromator is an optical device that is used to isolate a specific wavelength or range of wavelengths of light from a broader spectrum of light. It is typically used in spectroscopy, where the goal is to measure the intensity of light at a specific wavelength or over a range of wavelengths.
The resolution of a monochromator, Δλeff, is given by the equation: Δλeff = (D^-1) * (exit slit width)
Plugging in the given values:
Δλeff = (1.8 nm/mm) * (500 micrometers)
Converting micrometers to millimeters:
Δλeff = (1.8 nm/mm) * (0.5 mm)
Δλeff = 3.6 nm
Learn more on monochromator here;
https://brainly.com/question/917245
#SPJ1