Which statement illustrates the difference between a chemical reaction and a nuclear reaction?
1) A nuclear reaction releases more energy per gram and appears to violate the law of conservation of mass.
2) A nuclear reaction releases less energy per gram and appears to violate the law of conservation of mass.
3) A nuclear reaction releases more energy per gram but does not appear to violate the law of conservation of mass.
4) A nuclear reaction releases less energy per gram but does not appear to violate the law of conservation of mass.
Answers
Nuclear reactions alter the nucleus of an atom, typically creating a new element. On the reverse hand, nuclear alterations are not involved in chemical reactions; all that happens is that the electrons are rearranged.
What distinguishes compound nuclear reactions from direct nuclear reactions?The quick direct reactions only involve a single nucleon contact. A perfect thermal equilibrium (similar energy distribution between nucleons) occurs inside a complex nucleus as a result of many nucleon-nucleon interactions—indeed, a great number of them.
How are the rates of nuclear and chemical reactions different?Nuclear decay rates are set but chemical reaction rates change depending on the conditions of the reaction. The time it takes for half of a radioisotope sample to decay is known as its half-life. Half of the atoms inside a nuclear material have decayed after one half-life, while the other half have not.
To know more about electrons visit:
https://brainly.com/question/18247399
#SPJ1
Related Questions
for the following equilibrium, if hcl is added, how will the quantities of each component change? alpo4(s)↽−−⇀al3 (aq) po3−4(aq)
Answers
The chemical reaction given is:AlPO4 (s) ↔ Al3+ (aq) + PO34- (aq)What will happen if HCl is added to the given equilibrium.
The addition of HCl causes a change in the equilibrium because HCl dissociates into H+ and Cl- ions, and these H+ ions react with PO34- ions. The reaction goes in the forward direction to consume H+ ions, producing more Al3+ and PO34- ions. Here is the balanced chemical equation:HCl (aq) + PO34- (aq) ↔ HPO32- (aq) + Cl- (aq)So, as HCl is added, it will react with PO34- ions, reducing their concentration. Therefore, to compensate, the equilibrium will shift to the right to produce more PO34- ions. This, in turn, will shift the equilibrium to produce more Al3+ ions as well, as per the following equation:AlPO4 (s) + HCl (aq) ↔ Al3+ (aq) + PO34- (aq) + H2O (l)As a result, the quantities of Al3+ and PO34- will increase, while the concentration of AlPO4 will decrease. The addition of HCl will result in an increase in the concentration of both Al3+ and PO34- ions while the concentration of AlPO4 will decrease.
To know more about chemical reaction , visit ;
https://brainly.com/question/11231920
#SPJ11
What is the percent sodium in sodium chloride?
Answers
The total mass of sodium chloride is 58.44 g/mol.
The mass of sodium is 22.99 g/mol.
To find the percent sodium in sodium chloride can be found by dividing the amounts.
\(\frac{22.99}{58.44}\approx0.39\)Therefore, the percent sodium is 39%.
which country produces the most carbon emissions total? How does this compare to its per capita carbon emissions?
Answers
China has the highest per capita carbon emissions worldwide, at 35.6 metric tons per person. Numerous countries in the Middle East have high levels emissions, particularly when compared to countries in Africa.
How does China approximate to its per capita carbon emission?The average Chinese person radiates quite a bit less than the average American. In 2019, China's per capita emissions reached 10.1 tons. By comparing, the US reached 17.6 tons, according to the Rhodium Group.
What causes carbon emission?Most carbon emissions are due to the use of fossil fuels, mostly for generation of electricity and heat, transportation, and manufacturing. Land use and forestry is another reason of carbon emissions, mostly due to deforestation.
To know more about carbon, visit here:
https://brainly.com/question/22530423
#SPJ1
write the chemical reaction and return the chemical equation: Ammonia when it interacts with oxygen produces nitric oxide (II) and water
Answers
Answer:
NH3 + 02 > NO + H2O
Explanation:
Jonathan used the following soil triangle to identify a sample of soil as sandy clay. Which description of soil likely allowed Jonathan to make this identification.
A. Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt.
B. Mostly large grains, with a gritty texture, 45% sand, 5% clay, 45% silt.
C. Mostly small grains, with a smooth texture, 30% sand, 5% clay, 65% silt.
D. Mostly small grains, with a sticky texture, 30% sand, 50% clay, 20% silt.
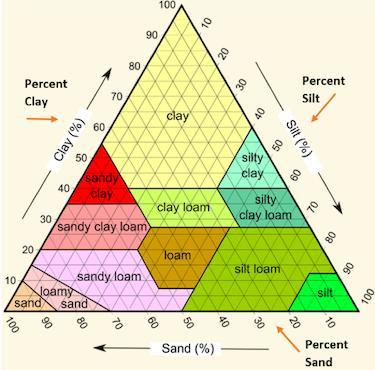
Answers
Answer:
Mostly large grains, with a sticky texture, 55% sand, 40% clay, and 5% silt
Explanation: I took the test, hope it helps.
Mostly large grains, with a sticky texture, 55% sand, 40% clay, 5% silt. The correct option is A.
What is soil triangle?One side of the triangle represents sand, the other side represents clay, and the third side represents silt.
A soil texture triangle is used to classify a soil's texture class. The percentages of sand, silt, and clay are scaled on the sides of the soil texture triangle.
Clay percentages are read across the triangle from left to right. Silt is read from top to bottom, upper right to bottom.
Jonathan used the soil triangle to identify a sample of soil as sandy clay; the soil description that allowed Jonathan to make this identification is 55% sand, 40% clay, and 5% silt, with mostly large grains and a sticky texture.
Thus, the correct option is A.
For more details regarding soil triangle, visit:
https://brainly.com/question/1698808
#SPJ2
5) How many Kilograms are in 12 L of Chlorine Gas?
Answers
Answer: 0.03798 kilograms
Explanation:
According to avogadro's law, 1 mole of every substance occupies 22.4 L at STP and contains avogadro's number \(6.023\times 10^{23}\) of particles.
To calculate the moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given volume}}{\text {Molar Volume}}=\frac{12L}{22.4L}=0.535moles\)
1 mole of chlorine gas \((Cl_2)\) weighs = 71 g
Thus 0.535 moles of chlorine gas \((Cl_2)\) will weigh = \(\frac{71}{1}\times 0.535=37.98g=0.03798kg\) (1kg=1000g)
Thus there are 0.03798 kilograms in 12 L of chlorine gas.
9. An element Q with electronic configuration 2.8, 2 reacts with elements R. with electronic configuration 2, 8, 7 to form an ionic compound. The likely formula of this compound is:
Answers
Answer:
QCl2
Explanation:
Q should belong to group 2 so valency is 2
R should belong to group 17 so valency is 1
ethane and ethene are both reacts with water and sulfuric acid as catalyst. what are the resulting products?
Answers
Ethanol is produced when ethane and ethene react with water and a catalyst like sulfuric acid. Adding concentrated sulfuric acid to hot ethanol (acts as a catalyst).
To eliminate carbon dioxide and sulphur dioxide that are created as byproducts, the gases are passed through a sodium hydroxide solution. The main product that is gathered over water is ethene. As a result, dehydration of ethanol produces ethene rather than ethane. The names Mattling acid and Oil of Vitriol are other names for sulfuric acid. It is highly caustic and acidic in nature. It dehydrates and oxidises when present in higher amounts. It is a clear, syrup-like liquid with no colour or smell. A substance having the chemical formula C 2H 6, ethane is an organic chemical.
Learn more about ethanol here
https://brainly.com/question/25002448
#SPJ4
is 2Sb+3L2-> 2SbL3 endothermic or exothermic?
Answers
Answer:
try working out the bond enthalpies for both sides of the equation :)
Explanation:
A bag contains exactly 22 solid-colored buttons: 4 red, 6 blue, and 12 white. What is the probability of randomly selecting 1 button that is not white?
Answers
Answer:
0.45
Explanation:
We can calculate the probability of randomly selecting one button that is not white by using the formula:
Number of non-white buttons / Total Number of buttons.
We can calculate the number of non-white buttons with the information given by the problem:
# Non-White buttons = 4 + 6 = 10
Now we calculate the probability:
10 / 22 = 0.45
The probability is 0.45, or a 45% chance.
sulfuric and nitric acids are the chemicals involved in acid deposition
Answers
Acid deposition is the deposition of acidic or acid-forming compounds from the atmosphere into the soil, water, and vegetation, which can lead to significant ecological harm.
The chemicals involved in acid deposition are sulfuric and nitric acids. These acids are formed from the emissions of sulfur dioxide (SO2) and nitrogen oxides (NOx) released from the burning of fossil fuels such as coal, oil, and gas.Content loaded sulfuric and nitric acids can react with water vapor in the atmosphere to form sulfuric acid (H2SO4) and nitric acid (HNO3). These acids can be carried by the wind for hundreds of kilometers from their source and deposited onto the earth's surface. The acid deposition can cause a wide range of ecological problems, including the death of fish and other aquatic organisms, the destruction of forests, and the degradation of soil quality.
The problem of acid deposition can be solved by reducing the emissions of sulfur dioxide and nitrogen oxides from the burning of fossil fuels. The adoption of cleaner energy technologies, such as wind and solar power, can help reduce these emissions. In addition, other measures such as the use of scrubbers on power plants to reduce SO2 emissions and the use of catalytic converters on cars to reduce NOx emissions can help to reduce acid deposition.
To know more about Acid deposition visit:
https://brainly.com/question/32219108
#SPJ11
A process that is approaching equilibrium will have a ______ δsuniv value.
Answers
A process that approaching equilibrium will have a constant universal value.
Equilibrium constant value is the ration of the concentration of the product over reactant. we can use the value of K to predict whether the reactant and product of the reaction are at equilibrium or not. When a reaction approach equilibrium both the forward and reverse reaction are occuing. At equilibrium, the foreward and reverse reaction are at the same rate. That means when the has reached a point where the concentration of the reactant and product remains unchanged with time. It is because both the reaction have same rate. The value of the equilibrium is the ratio of the concentration of the product over the reactant.
To learn more about Rate of reaction please visit:
https://brainly.com/question/12904152
#SPJ4
Q4
Which of the following is an example of nonpoint source pollution?
A. industrial effluent
B. Agricultural runoff
C. illegal dumping of waste
D. Oil leaking from your car
Answers
Answer:
Option C.
Ilegal dumping of waste
Explanation:
This is because non point source of pollution refers to source of pollution that are many and not directly one which is illegal or does not meet the legal term. This type of pollution does not have a point source, it has many sources and this type of pollution is cause by rainfall or precipitation. Where when the rain fall, it wash away the waste through to water bodies, causing pollution and endangering water bodies.
what happens when you add metal to carbonate
Answers
1What is one major difference between fossil fuels and biofuels?
ABurning fossil fuels releases greenhouse gases; burning biofuels releases no greenhouse gases incorrect answer
B Fossil fuels exist in unlimited supply; our supply of biofuels may run out someday incorrect answer
C Fossil fuels are very expensive to manufacture; biofuels are very inexpensive to manufacture incorrect answer
D You can drive farther on a single tank of fossil fuel than you can on a single tank of biofuel
Answers
The major difference between fossil fuels and biofuels is you can drive farther on a single tank of fossil fuel than you can on a single tank of biofuel. The correct option is D.
What are fossil fuels?A fossil is a naturally extracted fuel, for example, the anaerobic decompression of buried dead animals, while biofuel is any biomass fuel, plant or algae, or animal waste. Since these materials can quickly be replenished, biofuels are considered renewable energy sources.
Biofuels are created from renewable feedstocks, unlike fossil fuels, which are finite resources. As a result, their manufacturing and use might theoretically continue forever.
Therefore, the correct option is D. You can drive farther on a single tank of fossil fuel than you can on a single tank of biofuel.
To learn more about fossil fuels, refer to the link:
https://brainly.com/question/3371055
#SPJ2
what conversion factor is needed to calculate the number of atoms in 8.6 moles of Aluminum?
A. 1 mole/ 6.02x10^23 atmos
B. 6.02X10^23 atoms/ 1 mole
C. 1 mole/ 26.98zg
D. 26.982g/ 1 mole
Answers
Answer:hey did you ever get an answer? I need it for this exam
Explanation:
in a titration, 354 ml of 0.21 m formic acid hcooh was added to 126 ml of 0.9 m naoh. what will be the ph at that point in the titration?
Answers
At the point in the titration where 354 ml of 0.21 M HCOOH was added to 126 ml of 0.9 M NaOH, the pH is approximately 1.67.
To find the pH at the point in the titration where 354 ml of 0.21 M HCOOH was added to 126 ml of 0.9 M NaOH, we can use the following steps:
Write the balanced chemical equation for the reaction between formic acid and sodium hydroxide:
HCOOH(aq) + NaOH(aq) → O(l) + CO(g) + NaOH(aq)
Use the volume of the unknown acid solution (354 ml) and the volume of NaOH solution needed to neutralize it (126 ml) to find the concentration of formic acid:
[HCOOH] = [HCOOH] x V
[HCOOH] = 354 ml x 0.21 M
[HCOOH] = 77.6 mM
Use the molarity of the formic acid and the volume of NaOH solution to find the concentration of NaOH:
[NaOH] = [NaOH] x V
[NaOH] = 126 ml x 0.9 M
[NaOH] = 115.6 mM
Use the concentrations of the acid and base to find the stoichiometric equation for the reaction:
[HCOOH] = [NaOH] x (1 + [HCOOH]/[NaOH])
[HCOOH] = 77.6 mM x (1 + 77.6 mM/115.6 mM)
[HCOOH] = 80.4 mM
Use the balanced stoichiometric equation and the volumes of the acid and base to find the change in volume of the solution during the titration:
ΔV = [HCOOH] x V_initial - [HCOOH] x V_final
ΔV = 80.4 mM x 354 ml - 80.4 mM x 126 ml
ΔV = 1284 ml - 1056 ml
ΔV = 228 ml
Finally, use the change in volume to find the volume of NaOH solution needed to neutralize the formic acid:
ΔV_NaOH = -ΔV
ΔV_NaOH = -228 ml
ΔV_NaOH = 228 ml
V_NaOH = -228 ml
V_NaOH = 228 ml
Therefore, at the point in the titration where 354 ml of 0.21 M HCOOH was added to 126 ml of 0.9 M NaOH, the pH is approximately 1.67.
Learn more about titration visit: brainly.com/question/13307013
#SPJ4
a gas at constant volume has a pressure of 3.20 atm at 300. k. what will be the pressure of the gas at 290. k? 2.86 atm 3.09 atm 3.31 atm 3.56 atm
Answers
The relationship between pressure and temperature of a fixed amount of gas in a rigid container is called Charles’ Law.
According to Charles’ Law, for a given mass of gas at a constant volume, the volume of the gas varies directly with the temperature. It can be represented by the formula :V/T = constant where, V = volume of the gas T = temperature of the gas (in Kelvin)constant = proportionality constant Since pressure, volume, and temperature of the gas are interdependent, we can write:
PV/T = constant. We can use this formula to solve the problem. We know that the volume of the gas is constant. So, we can write:
P1/T1 = P2/T2 where, P1 = 3.20 atm (pressure at 300 K)T1 = 300 K (temperature at 3.20 atm)T2 = 290 K (temperature at unknown pressure)
Now, we can calculate P2 (pressure at 290 K) as:
P2 = P1 × (T2/T1) = 3.20 atm × (290 K/300 K) = 3.09 atmAnswer:3.09 atm
When the temperature of a fixed amount of gas is increased, its volume also increases. Similarly, when the temperature is decreased, the volume also decreases. This relationship between the volume of a gas and its temperature at a constant pressure is called Charles’ Law. It can be stated as:
V/T = constant, where V is the volume of the gas and T is its temperature in Kelvin. The proportionality constant in the above equation is the number of moles of the gas multiplied by the gas constant (R).
Mathematically, we can represent this relationship between pressure, volume, and temperature of a gas as: PV/T = constant.
When the volume of the gas is constant, the above equation becomes:
P1/T1 = P2/T2where P1 and T1 are the initial pressure and temperature of the gas, and P2 and T2 are the new pressure and temperature of the gas, respectively.
Using this equation, we can calculate the pressure of the gas at a new temperature, provided we know its initial pressure and temperature, and the new temperature.
Therefore, the pressure of the gas at 290 K will be 3.09 atm.
To know more about Charles’ Law visit:
brainly.com/question/27820267
#SPJ11
14. Iron ions bond with in solution to produce a purple color. O acetylsalicylic acid o acetic acid Osalicylic acid tio water
Answers
Osalicylic acid
Osalicylic acid, also known as 2-hydroxybenzoic acid, is a chemical compound that can bond with iron ions in solution, resulting in a purple color.
Iron ions, in the presence of Osalicylic acid, form a complex known as a chelate. This complex is characterized by the coordination of the iron ion with the Osalicylic acid molecule, creating a stable structure. The formation of this chelate is responsible for the observed purple color.
When Osalicylic acid is added to a solution containing iron ions, the hydroxyl group (-OH) of Osalicylic acid can donate a lone pair of electrons to the iron ion, forming a coordinate bond. This coordination causes a shift in the energy levels of the electrons within the complex, resulting in the absorption of light in the visible spectrum. The absorbed light corresponds to the complementary color of purple, giving the solution its distinctive color.
In summary, when iron ions bond with Osalicylic acid in solution, they form a chelate complex that absorbs light in the visible spectrum, resulting in a purple color. This phenomenon is commonly used in analytical chemistry for the detection and quantification of iron ions.
Learn more about Osalicylic acid
https://brainly.com/question/33401227
#SPJ11
Identify the items needed to run a spectrophotometry experiment. - spectrophotometer - hot plate - blank solution - sample solutions - cuvette - stir bar
Answers
A spectrophotometry experiment involves spectrophotometer, blank solution ,sample solutions and cuvette .
What is spectrophotometer?
Spectrophotometry is a method to measure how much a chemical substance absorbs light by measuring the intensity of light as a beam of light passes through sample solution. The basic principle is that each compound absorbs or transmits light over a certain range of wavelength.
This measurement can also be used to measure the amount of a known chemical substance. Spectrophotometry is one of the most useful methods of quantitative analysis in various fields such as chemistry, physics, biochemistry, material and chemical engineering and clinical applications.
Learn more about spectrophotometer,here:
https://brainly.com/question/24195565
#SPJ1
Mi One should know the properties of the components of the mixture to separate it. Explain with an example.
Answers
Can someone help mee ?

Answers
The quantity of refrigerant in a system is less critical when the system has a(n).
Answers
The quantity of refrigerant in a system is less critical when the system has increased operating pressures.
A low coolant level can cause the engine to overheat. This can cause the compressor not to start or the circuit breaker to trip prematurely. If left unchecked, the engine will eventually burn out and completely stall. Refrigerants with a low critical temperature have a large decrease in cooling performance.
The critical temperature of the refrigerant should be higher than the condensing temperature to obtain greater heat transfer at a constant temperature. When the air conditioner runs low on refrigerant, it loses its ability to transfer heat from within. This means that the air that's being blown into your AC coil isn't cooling properly, so your vents will start circulating warm air throughout your home.
Learn more about Refrigerant here:-https://brainly.com/question/26395073
#SPJ9
Which of the following happens when NaCl dissolves in water?
the chloride ions are attracted to the negative end of the water dipole
sodium and chloride ions form covalent bonds with the water molecules
the sodium ions are attracted to the negative end of the water dipole
the sodium ions are attracted to the positive end of the water dipole
both a) and d)
Answers
When NaCl dissolves in water, both a) the chloride ions are attracted to the negative end of the water dipole, and d) the sodium ions are attracted to the positive end of the water dipole. The correct option is E. both a) and d).
When NaCl (sodium chloride) is dissolved in water, it undergoes a process called dissociation, where the ionic compound breaks apart into its constituent ions: Na⁺ (sodium ions) and Cl⁻ (chloride ions). Water molecules, H₂O, are polar molecules due to the electronegativity difference between oxygen and hydrogen atoms.
The oxygen atom in water has a higher electronegativity and attracts electrons more strongly, resulting in a partial negative charge (δ-) on the oxygen atom and a partial positive charge (δ+) on the hydrogen atoms. This polarity leads to the formation of a dipole within the water molecule.
When NaCl dissolves in water, the positive sodium ions are attracted to the negative end of the water dipole. The partial negative charges on the oxygen atoms of water molecules are attracted to the sodium ions. This attraction between the positive and negative charges is an example of an ionic interaction.
Similarly, the negative chloride ions are attracted to the positive end of the water dipole. The partial positive charges on the hydrogen atoms of water molecules are attracted to the chloride ions.
As a result, both the chloride ions and the sodium ions interact with the water molecules through electrostatic forces, with the chloride ions attracted to the negative end and the sodium ions attracted to the positive end of the water dipole.
Therefore, the correct answer is option e) "both a) the chloride ions are attracted to the negative end of the water dipole, and d) the sodium ions are attracted to the positive end of the water dipole."
To know more about ionic compound refer here:
https://brainly.com/question/30420333#
#SPJ11
When NaCl dissolves in water, the sodium ions are attracted to the negative end of the water dipole, while the chloride ions are attracted to the positive end of the water dipole.
When NaCl (sodium chloride) dissolves in water, it undergoes a process called dissociation. In this process, the ionic bonds between the sodium and chloride ions in the solid NaCl are broken, and the individual ions become surrounded by water molecules.
The water molecules are polar, meaning they have a positive end (hydrogen) and a negative end (oxygen). When NaCl dissolves in water, the positive sodium ions (Na+) are attracted to the negative end of the water dipole (oxygen), while the negative chloride ions (Cl-) are attracted to the positive end of the water dipole (hydrogen).
This attraction between the ions and the water molecules allows the NaCl to dissolve in water and form a solution.
Learn more:
About NaCl here:
https://brainly.com/question/32275922
#SPJ11
A 60kg bucyclist going 2 m/s increased his output by 1800 j .what was his final velocity
Answers
Answer:
8
Explanation:
trust
question 70 52) if one strand of a dna molecule has the sequence of bases 5'-attgca-3', the other complementary strand would have the sequence a) 5-'taacgt-3'. b) 5'-tgcaat-3'. c) 5'-uaacgu-3'. d) 3'-uaacgu-5'.
Answers
If one strand of a DNA molecule has the sequence of bases 5'-ATTGCA-3', the other complementary strand would have the sequence 5'-TGCAAT-3'.
The complementary strand of DNA would have the sequence 5'-TGCAAT-3' if one strand of a DNA molecule has the sequence of bases 5'-ATTGCA-3'.There are a number of rules governing which bases pair with each other. In DNA, Adenine (A) pairs with Thymine (T), and Guanine (G) pairs with Cytosine (C).
Therefore, because A and T pair together and G and C pair together, the complementary strand can be found by simply switching out A for T and G for C or vice versa.
The base-pairing rules also imply that the 5' end of one strand is complementary to the 3' end of the other strand and vice versa. The correct complementary sequence to 5'-ATTGCA-3' would thus be 5'-TGCAAT-3'.
Therefore, option B) 5'-TGCAAT-3' is the right answer.
To learn more about complementary strand check the link below-
https://brainly.com/question/1534778
#SPJ11
A local plant nursery uses large sprinklers to water the plants twice a day. the water contains phosphorus, which is a fertilizer that helps plants grow. some of the water becomes runoff and ends up in nearby streams and lakes. this is an example of which type of short-term human-induced environmental change? eutrophication non-sustainable harvesting nonnative species introduction deforestation
Answers
Eutrophication
In an aged aquatic habitat like a lake, eutrophication is the progressive rise in the concentration of phosphorus, nitrogen, and other plant nutrients. As the volume of organic matter that can be converted into nutrients increases, the productivity or fertility of such an ecosystem also naturally rises.
What is Eutrophication ?Eutrophication may be caused by a number of things, including overuse of fertilisers, untreated sewage, the use of phosphorous-containing detergents, and industrial waste discharge.
Eutrophication naturally. Natural eutrophication is a process that develops in water resources over a very long period of time as a result of a slow buildup of nutrients and organic waste. Anthropogenic or cultural eutrophication.Learn more about Eutrophication here:
https://brainly.com/question/26956972
#SPJ4
with respect to chemical bonding, which particles play the most active role?
Answers
With respect to chemical bonding, electrons play the most active role. Electrons are the subatomic particles involved in the formation of chemical bonds between atoms. They determine the reactivity and chemical behavior of atoms by participating in the sharing, transfer, or redistribution of electrons.
In covalent bonding, atoms share electrons to achieve a more stable electron configuration. The sharing of electrons allows atoms to fill their valence shells and form stable molecules.
In ionic bonding, electrons are transferred from one atom to another, resulting in the formation of ions. The electrostatic attraction between the positively charged cations and negatively charged anions holds the ionic compound together.
In metallic bonding, electrons are delocalized or free to move throughout a metal lattice. The shared "sea" of electrons allows metals to conduct electricity and exhibit properties such as malleability and ductility.
Overall, electrons are the primary particles involved in chemical bonding, determining the strength, type, and properties of chemical bonds between atoms.
To know more about chemical bonding refer here
https://brainly.com/question/22661776#
#SPJ11
Consider the substitution reaction that takes place when (R)-3-bromo-3-methylhexane is treated with methanol. Which of the following would be true? A) The reaction would take place only with inversion of configuration at the stereogenic center. B) The reaction would take place only with retention of configuration at the stereogenic center. C) The reaction would take place with racemization. D) No reaction would take place. E) The alkyl halide does not possess a stereogenic center. a. А b. B c. C d. D e. Е
Answers
The reaction would take place with racemization. Option C.
The reaction between (s)-3-Bromo-3-methyl hexane and water is a nucleophilic substitution reaction as the leaving group present on the substrate is displaced by a nucleophile. Water is a polar protic solvent and he prefers SN1 reactions over SN2 reactions. Therefore, the reaction mechanism is SN1.
The carbocation and its substituents are all in the same plane. In other words, the nucleophile can attack from either side. As a result, both enantiomers are formed in the SN1 reaction, producing a racemic mixture of both enantiomers. Alkenes react with pure liquid bromine at low temperatures or with solutions of bromine in organic solvents such as carbon tetrachloride. The double bond is broken and a bromine atom is attached to each carbon atom.
Learn more about Methylhexane here:-https://brainly.com/question/28295748
#SPJ4
How many spaces have been left for the possible discovery of new elements?
Answers
this is the answer to ur question hope is helpful
