why can you make a cup of hot tea sweeter than a cup of iced tea? responses hot tea does not become saturated. hot tea does not become saturated. hot tea is capable of dissolving more sugar than iced tea. hot tea is capable of dissolving more sugar than iced tea. iced tea is capable of dissolving more sugar than hot tea. iced tea is capable of dissolving more sugar than hot tea. iced tea is already saturated.
Answers
Answer:
Hot tea is capable of dissolving more sugar than iced tea.
Explanation:
Related Questions
A cube of metal has a mass of 5.05 x 10°g and its density is known to be 12.77 g/mL, what is the volume of this metal?
A 395.5 mL
B. 2.529 mL
C. 63,910 mL
D. 64.48 ml
Answers
Answer:
Explanation:
option A is correct
A glass of water left in the sun becomes warm. Estella adds ice to the water to make it cool. What energy change occurs when ice is added to the warm drink?
answer choices
The water loses heat to the ice, causing the ice to melt.
The water absorbs the cold temperature from the ice.
Since the ice is cold, it adds cold to the environment.
The melting ice causes the water to expand, cooling it.
Answers
The energy change that occurs when ice is added to the warm drink when a glass of water left in the sun becomes warm and Estella adds ice to the water to make it cool is that the water loses heat to the ice, causing the ice to melt.
The correct option is A.
What causes ice to melt in warm water?Ice melts in warm water due to the transfer of heat energy from the water to the ice.
Heat flows from a warmer object to a cooler object until they reach thermal equilibrium, meaning they are at the same temperature. In the case of ice melting in warm water, the water is warmer than the ice, so heat flows from the water to the ice, causing the ice to melt.
Learn more about thermal energy at: https://brainly.com/question/19666326
#SPJ1
Determine the charge of each ion.
al oxygen ion with 10 electrons
(b) aluminum ion with 10 electrons
(c) titanium ion with 18 electrons
(d) iodine ion with 54 electrons
Answers
Answer:
(a) ==> O²-
(b) ==> Al³+
(c) ==> Ti²+
(d) ==> I-
i need help with my science homework on the last question pleasee!! it’s due tomorrow.

Answers
Answer:
I hope this helps
Please mark brainliest
Explanation:
Change in those habitats affects the organisms living there. Species can change over time in response to changes in environmental conditions through adaptation by natural selection acting over generations. Traits that support successful survival and reproduction in the new environment become more common.
calculate the molar heat of solution of potassium chlorate is 41.4
Answers
When, molar heat of solution of potassium chlorate is +41.4 kJ/mol. The temperature of the water will decreases when, potassium chlorate is dissolved into it.
When potassium chlorate is dissolved in water, the process is typically exothermic, meaning it releases heat. In this case, since the molar heat of solution of potassium chlorate is specified as +41.4 kJ/mol, it indicates that the dissolution process is endothermic, and 41.4 kJ of heat is absorbed per mole of potassium chlorate dissolved.
Therefore, when potassium chlorate is dissolved in water, the temperature of the water will decrease. The heat energy is transferred from the water to the potassium chlorate, resulting in a decrease in the temperature of the water.
To know more about potassium chlorate here
https://brainly.com/question/15979985
#SPJ4
--The given question is incomplete, the complete question is
"The molar heat of solution of potassium chlorate is +41.4 kJ/mol. What will happen to the temperature of a sample of water as potassium chlorate is dissolved into it?"--
A gas is composed of individual particles which are in constant?
Answers
Answer:
Gases consist of particles (molecules or atoms) that are in constant random motion. Gas particles are constantly colliding with each other and the walls of their container. These collisions are elastic; that is, there is no net loss of energy from the collisions.
Explanation:
hope it helps
Which functional group is represented in the diagram below?

Answers
Answer: Ketone
Explanation:
how to make a pb&j in 30 steps
Answers
this was great and fun to do

when aqueous solutions of sodium phosphate and copper(ii) nitrate are combined, solid copper(ii) phosphate and a solution of sodium nitrate are formed. the net ionic equation for this reaction is:
Answers
When aqueous solutions of sodium phosphate and copper (II) nitrate are combined, solid copper (II) phosphate and a solution of sodium nitrate are formed.
The net ionic equation for this reaction is written as follows:
Cu²⁺(aq) + PO₄³⁻(aq) → Cu₃(PO₄)₂(s)
It is important to note that the net ionic equation shows only the species (atoms, ions, or molecules) that undergo changes during the chemical reaction.
The complete balanced chemical equation for the reaction is:
Na₃PO₄(aq) + 3Cu(NO₃)₂(aq) → Cu₃(PO₄)₂(s) + 6NaNO₃(aq)
Let's write the ionic equation. Na₃PO₄ dissociates into Na⁺ and PO₄³⁻ in an aqueous solution while Cu(NO₃)₂ dissociates into Cu²⁺ and 2NO₃⁻ in an aqueous solution.
Ionic equation:
3Cu²⁺(aq) + 2PO₄³⁻(aq) → Cu₃(PO₄)₂(s)
We can see that the Na⁺ and NO₃⁻ ions are spectator ions in the reaction, that is why we call it a net ionic equation. This equation shows only the ions that participate in the reaction.
Learn more about the Ionic equation:
https://brainly.com/question/13879496
#SPJ11
Endothermic reactions are different from exothermic reactions because :
A. The temperature of the reaction drops.
B. The temperature of the reaction increases
C. The temperature of the reaction stays the same.
Answers
Answer:
I don't think endothermic reactions have anything to do with temperature. Instead they focus on the direction of the transfer of energy whether it is inside a body or to the surroundings.
IS
The system below was at equilibrium and
then some O2 gas was added to the
container. What change will occur for the
system?
2SO2(g) + O₂(g) = 2SO3(g) + 198 kJ
A. The reaction will shift toward the reactants (left) and
increase the concentrations of SO₂ and O₂.
B. The reaction will shift toward the reactants (left) and
decrease the concentration of SO3.
C. The reaction will shift toward the products (right) and
decrease the concentration of SO2.
D. The reaction will shift toward the products (right) and
increase the concentration of SO2.

Answers
Answer:
C
Equilibrium shifts to the right, as more SO₃ are formed, the products are being used up. Hence, the concentration of SO₂ decreases!
I hope this helps!
11. A 150 gram sample of radon-222 goes through alpha decay. The half-life of
radon-222 is 3.82 days. How much radon will be left after 3.82 days?
Answers
Answer:
The correct answer is - 75 grams.
Explanation:
Half-life is the amount of time that is used by the given amount of substance or element to make it half of the initial amount of a particular radioactive substance.
Equation for alpha decay for radon
\(^222_{86}Rn\Rightarrow^{218}_{84}Po \)
we need to calculate the left amount of radon after given time
using formula half life
\(N(t)=N_{0}e^{-\lambda t}\)
\(\lambda =\dfrac{0.693}{3.82}\)
\(\lambda=0.1814\)
\(N(3.82)=150\times e^{-0.1814\times3.82}\)
\(N(3.82)=75.0\ g\)
Thus, the left amount of radon is - 75 grams.
a solution is prepared by adding 0.10 mol of lithium nitrate, lino3, to 1.00 l of water. which statement about the solution is correct? a) the solution is basic. b) the solution is neutral. c) the solution is strongly acidic. d) the solution is weakly acidic. e) the values for ka and kb for the species in solution must be known before a prediction can be made
Answers
The correct option is (b) the solution is neutral. The solution is prepared by adding 0.10 mol of \(LiNO_3\) to 1.00 L of water is a neutral solution.
Lithium nitrate (\(LiNO_3\)) is an ionic compound that dissociates into ions when dissolved in water:
\(LiNO_3\) → \(Li^+\) (aq) + \(NO_3^-\)(aq)
\(Li^+\) and \(NO_3^-\) are both spectator ions, which means they don't participate in acid-base reactions. Therefore, the acidity of the solution will be determined by the reaction between water and the remaining ions.
Since neither \(Li^+\) nor \(NO_3^-\) reacts with water, the solution will be neutral. It is important to note that the \(K_a\) and \(K_b\) values of the species in solution do not need to be known in order to predict the acidity of the solution, since \(Li^+\) and \(NO_3^-\) are both spectator ions and do not participate in acid-base reactions.
Therefore, the correct option is (b) the solution is neutral.
for more such question on neutral solution
https://brainly.com/question/9395871
#SPJ11
how many grams of aluminum chloride are produced when aluminum reacts with 18.0 grams of hydrochloric acid to produce aluminum chloride and hydrogen gas?
Answers
There 88.94 grams would be grams of aluminum chloride are produced when aluminum reacts with 18.0 grams of hydrochloric acid to produce aluminum chloride and hydrogen gas.
To calculate aluminum chloride that are produce first we should write the balance reaction
2Al + 6HCl → 2AlCl₃ + 3H₂,
Then we can calculate the moles of aluminum
Moles aluminum = mass/ mass molar
Moles aluminum = 18 grams / 27 grams / moles
Moles aluminum = 0.667 moles
Form the reaction, it is clear that 2.0 moles of Al react with 6.0 mole of HCl to produce 2.0 moles of AlCl₃ and 3.0 mole of H₂.
using unitary method we can calculate the moles of Aluminum chloride
moles AlCl₃ = 2/2 x mole Al
Moles AlCl₃ = 2/2 x 0.667 moles =0.667 moles
Mass AlCl₃ = moles x mass molar
Mass AlCl₃ = 0.667 moles x 133.34 g/mol
Mass AlCl₃ = 88.94 grams
learn more about mass at https://brainly.com/question/12676886
#SPJ4
27.20cm3 of a NaOH solution was titrated with 25cm3 of a 0.75mol/dm3 HNO3. What was the concentration of the NaOH solution
Answers
The Concentration of NaOH solution when NaOH solution was titrated with 25cm3 of a 0.75mol/dm3 HNO3 is 26.4 g/dm3.
Titration is a method of chemical analysis where the quantity of a sample's constituents is determined by adding a precisely measured amount of a different substance to which the desired constituent will react in a specific, known proportion.Given,
Volume of HNO3 is 25cm³ = 0.025 dm³
Amount of solute in mol = concentration in mol/dm3 × volume in dm3
Amount of HNO3 = 0.75 × 0.025 = 0.018 mol
The balanced equation is: NaOH(aq) + HNO3(aq) → NaNO3(aq) + H2O(l)
So the mole ratio NaOH:HNO3 is 1:1
Therefore 0.018 mol of NaOH reacts with 0.018 mol of HNO3.
Volume of sodium hydroxide solution 27.20cm³ =
0.027 dm³.
Concentration = 0.018 / 0.027 = 0.66 mol/ dm³
Relative formula mass of NaOH= 40
Mass = relative formula mass × amount
Mass of HCl = 40 × 0.66
= 26.4
So concentration = 26.4 g/dm3
Therefore, concentration of NaOH is 26.4 g/dm3
Learn more about Titration here:
https://brainly.com/question/186765
#SPJ9
Chuck wants to know how many electrons in an atom are not paired up. Which model would be best for Chuck to write
out?
a set of quantum numbers for the last electron in the atom
a configuration with numbers, letters, and superscripts
a dot structure of the atom
an orbital notation of the atom
Answers
Answer:
D. an orbital notation of the atom
Explanation:
Orbital notiation uses lines and arrows to show shells, subshells, and orbitals for electrons in an atom. Since it shows arrows being paired up in this diagram it would be the best model for Chuck to use.
On Edgenuity2020, the answer is D) an orbital notation of the atom
3. If I have 204 eggs how many dozens is that? ( 1 dozen = 12)
I KNOW THE ANSWER ! HELP ME SHOW MY WORK !
Answers
Answer:
17 dozens
Explanation:
204 divided by 12 equals 17 and to check your work multiply 17 by 12 and you get 204.
Why do you think it is difficult to tell that a plate beneath it moving right now
Answers
URGENT PLEASE ELPP!!!Calculate the density of a piece of metal that has a mass of 2.5 × 102 kg and a volume of 4.1 × 103 m3.
Answers
Answer:
Density =mass/Volume
First of all let's find the mass
= 2.5 ×102kg
= 225kg
Then the volume
=4.1 × 103 m3
=422.3m3
225kg /422.3m3
=0.53279659
The density of a piece of metal that has a mass of 2.5 × 102 kg and a volume of 4.1 × 103 m3 will be 6.03 kg/m³ .
What do you mean by the density of a substance ?
Density is defined as the ratio of mass of the object to the volume of the object. Different substances have different densities.
The mathematical expression for density is as follows:
Density=Mass/Volume
The mass of a substance is expressed in kilograms and the volume is expressed in liter.
Thus, the unit of density is kg L⁻¹.
The density of a substance does not depend on the amount of the matter.
Thus, density is an intensive property.
To calculate the density -:
Given,
Mass= 2.5 ×102 kg=2550 kg
Volume=4.1 × 103 m³=422.3m³
Density=mass/volume
Density=2550/422.3
Density=6.03kg/m³
Hence, 6.03kg/m³ is the density of a piece of metal .
Learn more about density ,here:
https://brainly.com/question/15164682
#SPJ5
How many moles of O2 are needed to produce 30 g of Fez0s?
Answers
The number of mole of oxygen gas, O₂ needed to produced 30 grams of Fe₂O₃ is 0.282 mole
How do i determine the number of mole of O₂ needed?First, we shall obtain the mole of 30 grams of Fe₂O₃. This is shown below:
Mass of Fe₂O₃ = 30 grams Molar mass of Fe₂O₃ = 159.69 g/mol Mole of Fe₂O₃ =?Mole = mass / molar mass
Mole of Fe₂O₃ = 30 / 159.69
Mole of Fe₂O₃ = 0.188 mole
Finally, we shall obtain the number of mole of O₂ needed. This is shown below:
4Fe + 3O₂ -> 2Fe₂O₃
From the balanced equation above,
2 moles of Fe₂O₃ were obtained from 3 moles of O₂.
Therefore,
0.188 mole of Fe₂O₃ will be obtain from = (0.188 × 3) / 2 = 0.282 mole of O₂
Thus, we can conclude that number of mole O₂ needed is 0.282 mole
Learn more about number of mole:
https://brainly.com/question/23350512
#SPJ1
Complete question:
How many moles of O₂ are needed to produce 30 g of Fe₂O₃?
does mitosis begin with a diploid cell?
Answers
Answer:
no
Explanation:
What do these two changes have in common?
using a large magnet to remove pieces of iron from a junkyard
newly poured concrete becoming hard
Answers
Answer: Both are only physical changes. •
Explanation:
How many feet are in 271 miles? Answer to the appropriate significant figures and show your work.
1 mi = 5280 ft
(show work)
Answers
Answer:
there is 5280 feet in one mile. times that by 271 and you get 1430880
Enter a complete ionic equation to show the reaction of aqueous Hg2(NO3)2 with aqueous sodium chloride to form solid Hg2Cl2 and aqueous sodium nitrate. Express your answer as a complete ionic equation. Identify all of the phases in your answer.
Answers
Answer: \(Hg_2^{2+}(aq)+2NO_3^-(aq)+2Na^+(aq)+2Cl^-(aq)\rightarrow Hg_2Cl_2(s)+2Na^+(aq)+2NO_3^-(aq)\)
Explanation:
A double displacement reaction is one in which exchange of ions take place. The salts which are soluble in water are designated by symbol (aq) and those which are insoluble in water and remain in solid form are represented by (s) after their chemical formulas.
A double displacement reaction in which one of the product is formed as a solid is called as precipitation reaction.
The balanced chemical equation is:
\(Hg_2(NO_3)_2(aq)+2NaCl(aq)\rightarrow Hg_2Cl_2(s)+2NaNO_3(aq)\)
The complete ionic equation will be :
\(Hg_2^{2+}(aq)+2NO_3^-(aq)+2Na^+(aq)+2Cl^-(aq)\rightarrow Hg_2Cl_2(s)+2Na^+(aq)+2NO_3^-(aq)\)
examples of land-use planning to limit mass wasting events include
Answers
Land-use planning is a crucial aspect of mitigating the negative impacts of mass wasting events.
Examples of effective land-use planning strategies include establishing building codes and zoning regulations in areas prone to landslides, ensuring proper drainage and erosion control measures are in place, and utilizing green infrastructure such as vegetative cover and retaining walls to stabilize slopes. Additionally, implementing early warning systems and educating the public about the dangers of mass wasting events can also aid in reducing the impacts of these natural disasters. Overall, proactive and comprehensive land-use planning is essential in limiting the damage caused by mass wasting events. These practices aim to protect property and lives by limiting the potential for mass wasting occurrences.
To know more about landslides visit:
https://brainly.com/question/13151615
#SPJ11
Which of the following equilibria would not be affected by a pressure change?
1) C2H4(g) + H2O(g) = C2H5OH(g)
2) 4HCl(g) + O2(g) = 2H2O(g) + 2Cl2(g)
3) PF3Cl2(g) = PF3(g) + Cl2(g)
4) CO(g) + H2O(g) = H2(g) + CO2(g)
Answers
Answer:
C2H4(g) +H2O(g)=C2H5OH(g)
Please help
How many moles of O2 are required to generate 12 moles SO2 gas? 2CuFeS2 + 502 → 2Cu + 2FeO + 4SO2 [ ? ] mol O₂ O2
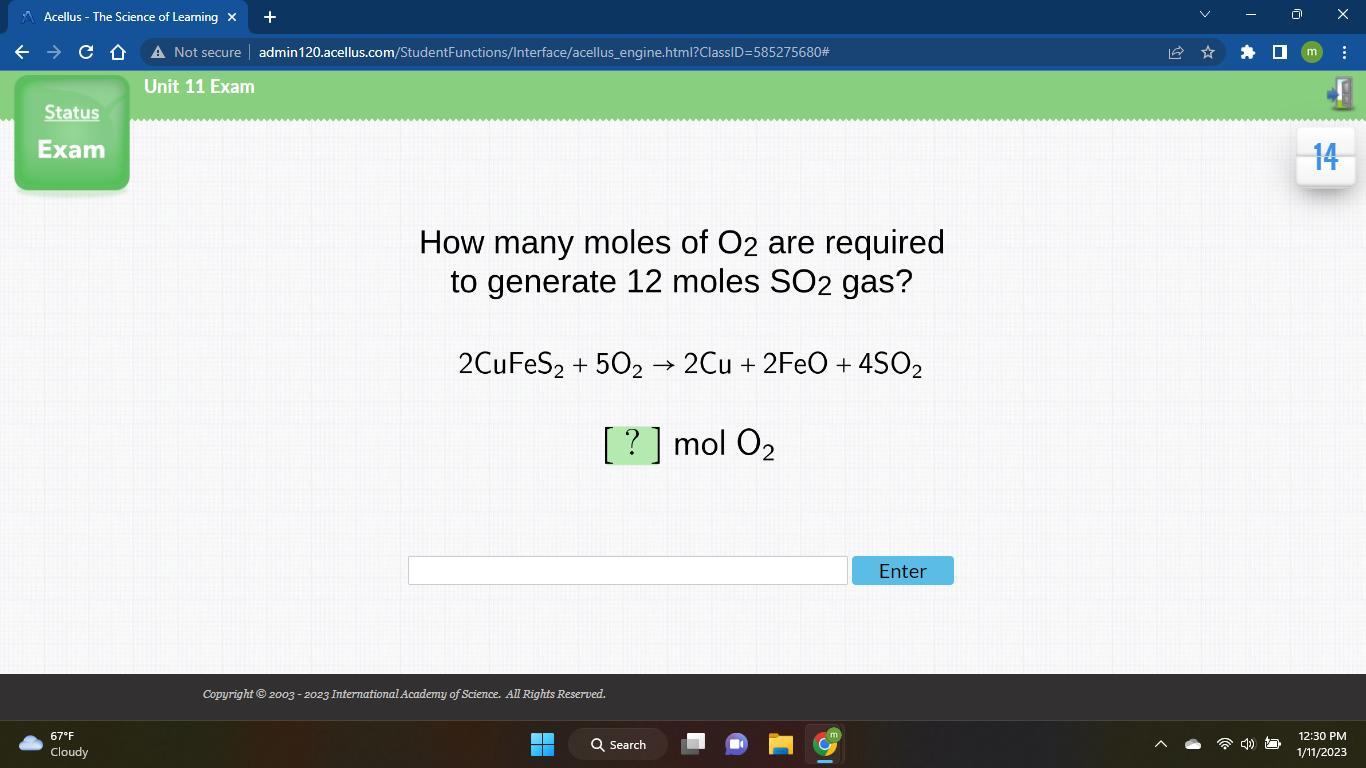
Answers
Answer:30 moles of oxygen are required if 12 moles of are consumed.
Explanation:The given balanced equation is:
From this balanced equation, there is 2:5 mol ratio between and .
We are asked to calculate the moles of required to react with 12 moles of .
It's a mol to mol conversion and the set up for this would be as:
=
So, 30 moles of oxygen are required to react with 12 moles of .
the type of change that results in a change in identity
Answers
Answer:
sorry read the question and answer the question
Help
Which of the following statements is true?
The number of electron shells in an atom is called the atomic number.
Boiling and melting points are chemical properties.
A molecule of oxygen can have atoms of carbon in it.
Elements are substances made of only one type of atom.
Answers
Answer:
A molecule of oxygen can have atoms of carbon in it.
Explanation: For CO2 there is one atom of carbon and two atoms of oxygen.
hope this help
plz mark brainliest
What is the difference between ethanol and methanol.