why would determining the heat capacity of an unknown be useful information?
Answers
Determining the heat capacity of an unknown material can be a useful tool for understanding its properties.
Heat capacity is a measure of the amount of heat energy required to raise the temperature of a material by a certain amount. By measuring the heat capacity of a material, scientists can gain insight into its thermal properties and understand how it will react to changes in temperature. For example, a material with a high heat capacity requires more heat energy to warm it up, but it will also take longer to cool off when the temperature is reduced. In addition, the heat capacity can be used to calculate the amount of energy needed to convert a material from solid to liquid or liquid to gas
Learn more about heat capacity here:
https://brainly.com/question/28302909
#SPJ4
Related Questions
How much energy does it take to boil 100 mL of water? (Refer to table of constants for water. )
A. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 6. 03 kJ/mol = 33. 5 kJ
B. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × (–285. 83 kJ)/mol = –1586 kJ
C. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 40. 65 kJ/mol = 226 kJ
D. 100 mL × 1g divided by 1mL × 1mol divided by 18. 02g × 4. 186 kJ/mol = 23. 2 kJ
Answers
Therefore, it takes approximately 23.2 kJ of energy to boil 100 mL of water.
The correct answer is D. 100 mL × 1g divided by 1mL × 1mol divided by 18.02g × 4.186 kJ/mol = 23.2 kJ
To calculate the energy required to boil 100 mL of water, we need to use the specific heat capacity of water, which is approximately 4.186 J/g·°C. The molar mass of water is 18.02 g/mol.
First, we convert the volume of water from milliliters to grams:
100 mL × 1 g/1 mL = 100 g
Then, we calculate the number of moles of water:
100 g × 1 mol/18.02 g = 5.548 mol
Finally, we multiply the number of moles by the molar heat of vaporization of water, which is approximately 40.65 kJ/mol:
5.548 mol × 4.186 kJ/mol = 23.2 kJ
Therefore, it takes approximately 23.2 kJ of energy to boil 100 mL of water.
Learn more about energy
https://brainly.com/question/8630757
#SPJ11
In nature, 78. 90% of the magnesium is magnesium-24, 10. 00% is magnesium–25, and 11. 10% is magnesium-26. Calculate the average atomic mass of magnesium in nature.
Answers
The atomic mass of an element is its average atomic mass given in atomic mass units (amu, also known as daltons, D). The atomic mass, which is a weighted average of all the isotopes of that element, is calculated by multiplying the mass of each isotope by its abundance.
Calculate the average atomic mass of magnesium in nature.
24.31 is the average atomic mass of Magnesium in nature.
The ratio of neutrons to protons varies among isotopes, even though they all come from the same family of elements. The quantity of protons an element has affects its atomic number on the Periodic Table.
The information supplied indicates that
The sum of the relative abundance percentages of each isotope is the atomic mass. Therefore
Mg's atomic mass = (23.98×78.6)+(24.98×10.11)+(25.98×11.29)/100
Mg's atomic mass = 18.85+2.53+2.93 = 24.31
To know more about average atomic mass , click on the link below:
https://brainly.com/question/28768877
#SPJ4
The average atomic mass of an element, expressed in atomic mass units, is the element's atomic mass (amu, also known as daltons, D). The mass of each isotope is multiplied by its abundance to determine the atomic mass, which is a weighted average of all the isotopes of that element.
What is the calculation of the Atomic mass?Magnesium has an average atomic mass of 24.31.
Despite belonging to the same family of elements, isotopes differ in the ratio of neutrons to protons. An element's atomic number on the Periodic Table is influenced by how many protons it contains.
The atomic mass is equal to the sum of the relative abundance percentages of each isotope. Thus, the atomic mass of magnesium
To know more Atomic Mass visit:
https://brainly.com/question/17067547
#SPJ4
B. which of the reef organisms are consumers?
Answers
Answer: The primary consumers are zooplankton, corals, sponges, Atlantic blue tang, and queen conch.
Explanation:
Which of the following molecules is NOT part of the thin filament?A. actinB. titinC. troponinD. tropomyosin
Answers
The molecule that is NOT part of the thin filament is titin.
The other molecules listed, actin, troponin, and tropomyosin, are all part of the thin filament.
Myofilaments mostly come in two varieties. The two types of filaments are thin filaments and thick filaments.
The thin filaments have a diameter of 7-9 nm. They are joined to the striated muscle's z discs.
Actin, troponin, and tropomyosin are the three proteins that make up each thin filament.
However, the primary protein in the thin filament is actin.
The helical strands of the thin filament, now known as F actin (being fibrous), are made up of 300–400 globular actin molecules that are joined end to end. During the contraction phase, a myosin cross-bridge or head can bind to each actin molecule. Troponin is an additional protein found in the thin filament.
To learn more about thin filament, visit:
https://brainly.com/question/30616312
#SPJ11
(110.3 g) + (45.27 g) + (54.43 g)
Answers
Answer:
210 grams
Explanation:
just add them all up
Built from amino acids
carry out most of the life-sustaining functions of the human body
Answers
Answer:
proteins carry out most of the life sustaining functions of the human body
Which choice represents a compound?Required to answer. Single choice.
(1.5 Points)
Cu
Fe
CO
Br
Answers
Answer:
CO
Explanation:
Elements will only one capital letter, that may/may not have a lower case letter after it. CO has a capital letter followed by another capital, so it's a compound
a similarly of chemical and physical change and a difference plssss help me
Answers
Answer:
Similarities: the states of the recants
Explanation:
Please answer question #4
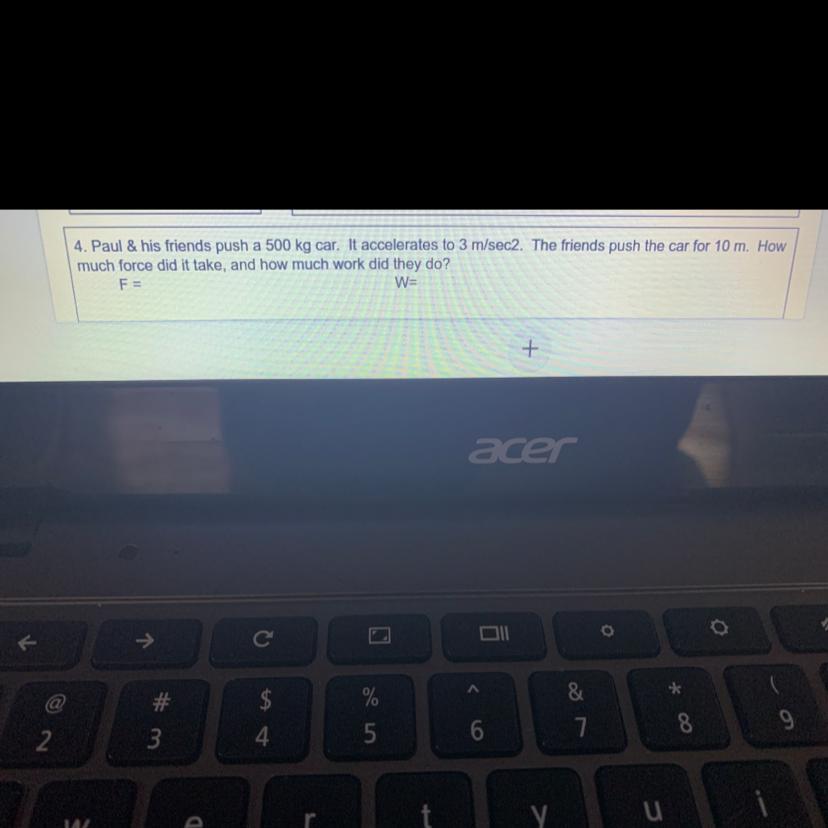
Answers
Answer:
F=500×3 = 1500 N
W = 1500×10 =15000 Nm
this question is giving me a hard time

Answers
Answer:
options 3rd is the correct answer
explain why reaction rates decline with time and use this information to correctly process the data (by choosing the proper data points to do linear regression)
Answers
Reaction rates decline with time because as the reaction progresses, the concentration of reactants decreases and the concentration of products increases. This means that there are fewer reactant molecules available to collide and react with each other, leading to a slower rate of reaction.
Additionally, the reaction may reach a state of equilibrium where the rate of the forward reaction is equal to the rate of the reverse reaction, resulting in no net change in concentrations of reactants and products over time.
To correctly process data for a reaction with declining rates, it is important to choose data points that reflect the initial, fast reaction rate before significant amounts of reactants have been consumed.
These points can be used to calculate the reaction rate constant and determine the order of the reaction. Using data points from later times when the reaction rate has slowed down may lead to incorrect calculations of the reaction rate and order.
To know more about the Reaction rates refer here :
https://brainly.com/question/13440548#
#SPJ11
student models the relationship between the earth and the sun using string and a ball. which of the following explains the relationship demonstrated?
Answers
The relationship demonstrated is the orbit of the Earth around the Sun. The ball represents the Sun and the string represents the gravitational force that keeps the Earth in its elliptical path around the Sun.
Relationship building is the process of establishing and maintaining relationships with people from and outside your network. Usually, people aim to build relationships with those who can help them achieve their goals or will support their mission.
Having strong relationship-building skills also means being able to approach and connect with others while keeping an open mind when communication difficulties arise.
Furthermore, it requires strong networking and teamwork skills, as they are necessary for all types of interpersonal communication.
And because relationship-building is considered a soft skill, we advise you to abstain from listing it in your resume’s skills’ section. Instead, show attention to detail and prove you’re a confident communicator who’s always up for a challenge.
To know more relationship demonstrated click this link-
brainly.in/question/26928952
#SPJ11
Given the following balanced equation, which is NOT a correct conversion factor?
4 NH3 + 5O2 → 4 NO + 6H₂O
a. 4 mol NH3 = 5 mol O2
b. 18.02 g H20 = 1 mol H2O
c. 5 mol O2 = 32.00 g 02
d. 5 mol O2 = 4 mol NO
e. 1 mol NH3 17.03 g NH3
Answers
The incorrect conversion factor is e. 1 mol NH3 = 17.03 g NH.
The balanced equation provided is:
4 NH3 + 5O2 → 4 NO + 6H2O
To identify the incorrect conversion factor, we need to analyze the stoichiometry of the reaction and compare it to the given options.
a. 4 mol NH3 = 5 mol O2
This is a correct conversion factor based on the balanced equation, where the stoichiometric ratio between NH3 and O2 is 4:5.
b. 18.02 g H2O = 1 mol H2O
This is a correct conversion factor based on the molar mass of water (H2O), which is approximately 18.02 g/mol.
c. 5 mol O2 = 32.00 g O2
This is a correct conversion factor based on the molar mass of oxygen gas (O2), which is approximately 32.00 g/mol.
d. 5 mol O2 = 4 mol NO
This is a correct conversion factor based on the stoichiometric ratio between O2 and NO in the balanced equation, which is 5:4.
e. 1 mol NH3 = 17.03 g NH
This conversion factor is incorrect. The molar mass of ammonia (NH3) is approximately 17.03 g/mol, not NH. The correct conversion factor should be 1 mol NH3 = 17.03 g NH3.
Therefore, the incorrect conversion factor is e. 1 mol NH3 = 17.03 g NH.
for more questions on conversion
https://brainly.com/question/30850837
#SPJ11
help me please guys #9

Answers
Answer:
Bases
Explanation:
Most soaps fall under the base category as they are slippery and will taste bitter if you taste it (DONT TRY IT THO).
what is the molar mass of citric acid (c6h8o7) and baking soda (nahco3)?
Answers
The molar mass of citric acid (c6h8o7) is 192.124g/mol
The molar mass of baking soda (nahco3) is 84.007g/mol
The molar mass of a chemical compound is defined as the mass of a sample of that compound divided by the amount of substance in that sample and is measured in moles. Molar mass is a mass property, not a molecular property of a substance.
Molar mass is the mass of 1 mole of the sample. To find the molar mass, add up the atomic masses (atomic weights) of all the atoms in the molecule. Use the masses listed in the periodic table or atomic weight table to determine the atomic mass of each element.
Learn more about molar mass here:https://brainly.com/question/15476873
#SPJ1
The molar mass of citric acid (C6H8O7) : 192.12 g/mol
The molar mass of citric acid (C6H8O7) : 84.007 g/mol
Baking soda is a monosodium salt of carbonic acid with alkalizing and electrolyte substitution properties. When dissociated, baking soda forms sodium and bicarbonate ions. Ion formation increases plasma bicarbonate and buffers excess hydrogen ion concentration, resulting in an increase in blood pH.
Citric acid is a naturally occurring weak acid in all citrus fruits. If you've ever sunk your teeth in a lemon, you've tasted citric acid. Manufacturers add artificial versions of it to processed foods. Drugs containing citric acid treat health problems such as kidney stones.
Learn more about molar mass here: https://brainly.com/question/837939
#SPJ4
A nucleus of uranium 235 decays. Its decay produces a nucleus of an unknown element (x), a nucleus of helium 4 and energy. What kind of radiation is produced by this decay?
Gamma beta or alpha?
Answers
Answer: Alpha
Explanation:
Alpha particle form of decay produces a nucleus similar to the element helium. During Alpha decay an atom spits out two protons and two neutrons from its nucleus.
How many atoms of each type are present in the empirical formula of Sample R0103?
Answers
The empirical formula of Sample R0103 is \(C_{12}H_{24}O_6\).
This is the molecular formula for a type of sugar called sucrose, which is commonly found in fruits, vegetables, and honey. It is a disaccharide, which Sucrose, a type of sugar that is found naturally in plants and is used as a sweetener in food and beverages. It is made up of 12 Carbon atoms, 24 Hydrogen atoms and 6 Oxygen atoms.The R0103 formula is an empirical formula used to calculate the amount of energy released in a given reaction. The formula uses the known energy content of the reactants and products to predict the energy released. The amount of energy released is determined by the difference between the energy content of the products and the energy content of the reactants.
learn more about sucrose Refer:brainly.com/question/28869238
#SPJ1
in the renal tubules, where is the na+/k+ pump located?
Answers
In renal tubules, renal Na-K-ATPase performs a key function withinside the energetic translocation of Na and K throughout this membrane.
In addition to withinside the "secondary energetic" shipping of some of different solutes. The Na-K pump actively transports sodium from the lumen of the renal tubule transcellularly into the interstitial fluid. The Na+K+-ATPase pump enables to keep osmotic equilibrium and membrane capability in cells. The sodium and potassium flow towards the awareness gradients. The Na+ K+-ATPase pump keeps the gradient of a better awareness of sodium extracellularly and a better degree of potassium intracellularly.
To learn more about renal tube check the link below:
https://brainly.com/question/9136517
#SPJ4
What is a stable electron configuration?
Answers
Enter the conjugate base for each acid.
H3PO4:H3PO4:
H2CO3:H2CO3:
CH3COOH:CH3COOH:
CH3NH+3:CH3NH3+:
Answers
Answer:
The conjugate base for each acid is obtained by removing a proton (H+) from the acid molecule. Here are the conjugate bases for the given acids:
H3PO4: H2PO4-
H2CO3: HCO3-
CH3COOH: CH3COO-
CH3NH+3: CH3NH2
Learn more about conjugate bases: https://brainly.com/question/18402797
#SPJ11
Propose Structures For Compounds That Fit The Following 'H NMR Data. 1) c7h14o 6h triplet at 0.9 δ 4h sextet at 1.6 δ 4h triplet at 2.4 δ
Answers
This compound is a quintet since it has five different types of protons (CH3, two types of CH2, CH, and the carbonyl proton).
In chemistry, a compound refers to a substance composed of two or more different elements that are chemically bonded together in fixed proportions. These elements combine through chemical reactions to form a compound with its own distinct properties and characteristics.
Compounds play a fundamental role in chemistry and are the building blocks of many substances found in nature and synthesized in laboratories. They can range from simple compounds, such as water or carbon dioxide, to complex molecules like proteins and pharmaceutical drugs.
It is important to distinguish compounds from mixtures, which are combinations of substances that are not chemically bonded. In a mixture, the different components retain their individual properties and can be separated through physical means, such as filtration or distillation. In contrast, compounds have unique properties that result from the chemical bonds between their constituent elements.
Based on the H NMR data provided, we can propose a structure for the compound as follows:
The presence of a triplet at 0.9 δ indicates the presence of a CH3 group. The 4H sextet at 1.6 δ indicates the presence of a CH2 group that is adjacent to another CH2 group. The 4H triplet at 2.4 δ indicates the presence of a CH2 group that is adjacent to a carbonyl group.
Therefore, a possible structure for the compound could be:
CH3-CH2-CH2-C(=O)-CH2-CH2-CH3
This structure has 7 carbons, 14 hydrogens, and 1 oxygen. It also has a triplet, a sextet, and a triplet in the H NMR spectrum, consistent with the given data.
This compound is a quintet since it has five different types of protons (CH3, two types of CH2, CH, and the carbonyl proton).
To know more about carbonyl visit:
https://brainly.com/question/23881479
#SPJ11
how many moles ofNaCl can be produced from 4.5 moles of na from 2Na+cl2->2Nacl
Answers
The number of moles of sodium chloride (NaCl) that can be produced is:
4.5 moles Na * (2 moles NaCl / 2 moles Na) = 4.5 moles NaCl from 4.5 moles of sodium (Na), you can produce 4.5 moles of sodium chloride (NaCl) according to the balanced chemical equation.
The balanced chemical equation shows that 2 moles of sodium (2Na) react with 1 mole of chlorine (Cl₂) to produce 2 moles of sodium chloride (2NaCl). From the given information, we have 4.5 moles of sodium (Na). According to the stoichiometry of the balanced equation, we can determine the number of moles of sodium chloride (NaCl) that can be produced by using a simple mole ratio. Mole ratio of Na to NaCl: 2 moles NaCl / 2 moles Na Therefore, the number of moles of sodium chloride (NaCl) that can be produced is: 4.5 moles Na * (2 moles NaCl / 2 moles Na) = 4.5 moles NaCl Hence, from 4.5 moles of sodium (Na), you can produce 4.5 moles of sodium chloride (NaCl) according to the balanced chemical equation.
For more question on moles
https://brainly.com/question/15356425
#SPJ11
Which of these is the source of the energy that plant cells use for photosynthesis?
Choose the correct answer.
Responses
radiant energy from the sun
radiant energy from the sun
kinetic energy from the wind
kinetic energy from the wind
potential energy from the soil
potential energy from the soil
mechanical energy from animals
Answers
Radiant Energy from the sun is the source of the energy that plant cells use for photosynthesis. The first response is the correct answer.
Nearly all of the energy on Earth comes from the sun. Through a process known as photosynthesis, it enables plants and other organisms to convert water and carbon dioxide into sugars.
The function of sunlight in photosynthesis is to supply energy. Heat and light are two different types of energy that the sun emits.
Chlorophyll can capture energy, which can then be converted into sugar during photosynthesis. Photosynthesis slows down when a plant receives insufficient sunlight. This implies that the plant's energy source—sugar—might not be getting to it in sufficient amounts. We can clearly see how crucial sunlight is to life.
To learn more about photosynthesis, refer to the answer below:
https://brainly.com/question/19160081
#SPJ1
what volume of water in ml initially at 85.4 C needs to be mixed with 200 ml of water initally at 29.5 C so that the final temperature of the water is 36.2 C
Answers
Answer:
About 27 mL of water.
Explanation:
We can use the heat transfer equation. Recall that:
\(\displaystyle q = mC\Delta T\)
Heat is transferred from the water with higher temperature to the water with lower temperature. Hence:
\(\displaystyle -q_1 = q_2\)
Substituting yields:
\(-m_1C \Delta T_1 = m_2C\Delta T _2\)
We can solve for m₁:
\(\displaystyle \begin{aligned} m_1 &= -\frac{m_2 C\Delta T_2}{ C \Delta T_1} \\ \\ & = -\frac{m_2\Delta T_2}{\Delta T _1}\end{aligned}\)
The desired final temperature of water is 36.2 °C. Substitute and evaluate:
\(\displaystyle \begin{aligned} m_1 & = -\frac{(200\text{ mL})(36.2\text{ $^\circ$C}-29.5\text{ $^\circ$C})}{(36.2\text{ $^\circ$C}-84.5\text{ $^\circ$C})} \\ \\ & = -\frac{(200\text{ mL})(6.7\text{ $^\circ$C})}{-49.2\text{ $^\circ$C}}\\ \\ & =27\text{ mL}\end{aligned}\)
In conclusion, about 27 mL of water should be added.
A particular experiment requires 13.5 µl hydrochloric acid solution. if 250 ml of hydrochloric acid solution is available, how many times can the experiment be performed? (note: 1ml = 1,000 µl.) about 18 about 18,500 about 18,500,000
Answers
Here, we're given a very simple unit conversion problem.
1 mL= 1 x 10⁻³ L
1 μL = 1 x 10⁻⁶ L
So, we can convert 250 mL and 13.5 μL into L as follows,
250 mL = 250 x 10⁻³ L
13.5 μL = 13.5 x 10⁻⁶ L
1 experiment requires 13.5 μL of HCl.
Hence,
the number of experiments = total volume / volume per experiment
= 250 x 10⁻³ L / 13.5 x 10⁻⁶ L
= 18518.518
= 18518
(number of experiments should be a whole number. Hence we have to choose 18518)
Hence, the number of experiments which can be performed is about 18500.
More about hydrochloric acidThere are various uses for hydrochloric acid. In addition to electroplating, it is utilized in the manufacture of chlorides, fertilizers, and dyes as well as in the textile, rubber, and photography industries.Hydrochloric acid is corrosive to mucous membranes, the skin, and the eyes. Acute (short-term) inhalation exposure may result in pulmonary edema, ocular irritation, and inflammation of the respiratory tract, nose, and eyes in people. The mucous membranes, esophagus, and stomach may corrode as a result of acute oral exposure, and cutaneous contact may result in severe burns, ulceration, and scarring in people. Gastritis, chronic bronchitis, dermatitis, and photosensitization have all been linked to chronic (long-term) occupational exposure to hydrochloric acid in employees.To view more about hydrochloric acid, refer to:
https://brainly.com/question/15231576
#SPJ4
The dog will eat more food to store fat and grow more hair.
The dog will lay in the sunlight to transpire
The dog will pant to circulate air throughout the body to cool
down
3.
The dog will grow a thick winter coat to keep warm during the
In the summer months, what is this dog's likely response to the rising temperatures?
summer
CLEAR ALL
Answers
hibernation exclamation mark
Mr block student are studying chemical
Answers
Answer:
egg
Explanation:
man
How many grams of water are produced, given that 6.8 moles of oxygen gas are produced as well?

Answers
Answer:
So 8 g of oxygen will react with 1 g of hydrogen to produce 9 g of water. 8–1 = 7 g of hydrogen ... Number of moles = given mass/ molecular or atomic mass.
Explanation:
chinese religions are ______, meaning that there is not necessarily any conflict in combining several different religious traditions or practices.
Answers
Answer:Buddhism
Explanation:
chinese religion is a Buddhism
remical Reactions 8.P.13 1 8 of 40
Which of the following is a correctly written chemical equation that demonstrates the conservation of mass?
O A H2 + O2 + H2O
O B. Mg + HCl + H2 + MgCl2
C. KC 103 → KCl + O2
D. H2O + CO2 + H2CO3
Answers
Answer:
H₂O + CO₂ → H₂CO₃
Option D is correct.
Law of conservation of mass:
According to this law, mass can neither be created nor destroyed in a chemical equation.
This law was given by French chemist Antoine Lavoisier in 1789. According to this law mass of reactant and mass of product must be equal, because masses are not created or destroyed in a chemical reaction.
Now we will apply this law to given chemical equations:
A) H₂ + O₂ → H₂O
There are two H and two O atoms present on left side while on right side only one O and two H atoms are present so mass in not conserved. This option is incorrect.
B) Mg + HCl → H₂ + MgCl₂
In this equation one Mg, one H and one Cl atoms are present on left side of equation while on right side two H, one Mg and two chlorine atoms are present. This equation also not follow the law of conservation of mass.
C) KClO₃ → KCl + O₂
There are one K, one Cl and three O atoms are present on left side of equation while on right side one K one Cl and two oxygen atoms are present. This equation also not following the law of conservation of mass.
D) H₂O + CO₂ → H₂CO₃
There are two hydrogen, one carbon and three oxygen atoms are present on both side of equation thus, mass remain conserved. This option is correct.