Answers
Related Questions
What is the density of krypton gas (MM = 83.8 g/mol) at 3.00
atm and 100. °C?
g/L
Answers
Answer:
8.21
Explanation:
Liquid/liquid and Acid/Base reactions are used in most organic reactions to ______.
Answers
Liquid/liquid and acid/base reactions are commonly used in organic reactions to catalyze or facilitate the reaction. Organic reactions are chemical reactions involving organic compounds, which are molecules that contain carbon and hydrogen atoms.
Many organic reactions require a catalyst or a facilitator to occur, and liquid/liquid and acid/base reactions are often used for this purpose.
In liquid/liquid reactions, two liquid phases are used, and the reactants and catalysts are distributed between them. This can provide a favorable environment for the reaction to occur, such as by increasing the solubility of the reactants or by separating reaction intermediates from the main reaction.
In acid/base reactions, an acid or a base is used as a catalyst or a facilitator. Acids can donate a proton (H+) to the reactants, while bases can accept a proton. This can change the electronic properties of the reactants and make them more reactive, or it can stabilize the reaction intermediates.
Learn more about organic reaction: https://brainly.com/question/30905538
#SPJ11
1) How many moles are in 3.24 x 1022 atoms of water?
Answers
Answer:
0.054 moles
Explanation:
It is the rounded off answer.

What is the general formula for a compound if anions occupy the hcp lattice points and cations occupy all the octahedral and tetrahedral holes?
a. AB
b. A3B
c. A2B
d. AB2
Answers
The general formula for a compound where anions occupy the hcp lattice points and cations occupy all the octahedral and tetrahedral holes is option (d) AB2.
In a hexagonal close-packed (hcp) lattice structure, anions occupy the lattice points, and cations can occupy both the octahedral and tetrahedral holes within the lattice. The arrangement of the cations in these holes depends on the ratio of cations to anions in the compound.
In this case, the general formula for the compound can be represented as AB2, where A represents the cation and B represents the anion. The number 2 in the formula indicates that each cation A is surrounded by two anions B in the lattice.
By selecting option (d) AB2, we imply that the cations occupy all the octahedral and tetrahedral holes in the hcp lattice, resulting in a compound with the specified arrangement of anions and cations.
To learn more about hexagonal close-packed (hcp) click here: brainly.com/question/32196960
#SPJ11
calculate the volume of concentrated sulfuric acid needed to 5.00 l of 10.0 m sulfuric acid. concentrated sulfuric acid has a molarity of 18.0 m and a density of 1.83 g/cm3 .
Answers
This yields a mass of 16.47 g of concentrated sulfuric acid.
What is sulfuric acid?Sulfuric acid, also known as oil of vitriol, is a strong mineral acid with the molecular formula H2SO4.
Volume of concentrated sulfuric acid needed = 5.00 L x (18.0 m / 10.0 m)
= 9.00 L
Mass of concentrated sulfuric acid needed = 9.00 L x 1.83 g/cm3
= 16.47 g
The volume of concentrated sulfuric acid needed to make 5.00 L of 10.0 m sulfuric acid can be calculated by multiplying the volume of the solution (5.00 L) by the molarity of the concentrated sulfuric acid (18.0 m) divided by the desired molarity of the solution (10.0 m). This yields a volume of 9.00 L of concentrated sulfuric acid. The mass of concentrated sulfuric acid needed can then be calculated by multiplying the volume of concentrated sulfuric acid (9.00 L) by the density of sulfuric acid (1.83 g/cm3). This yields a mass of 16.47 g of concentrated sulfuric acid.
To learn more about sulfuric acid
https://brainly.com/question/10220770
#SPJ4
procaine hydrochloride (mw = 272.77 g/mol) is used as a local anesthetic. calculate the molarity of a 3.548 m solution which has a density of 1.134 g/ml.
Answers
The molarity of the 3.548 m solution of procaine hydrochloride is 4.15 M. The molarity of the 3.548 m solution of procaine hydrochloride with a density of 1.134 g/ml can be calculated using the formula Molarity = (mass/volume) x (1/molecular weight).
First, we need to convert the density to mass/volume units, which is grams per liter (g/L). To do this, we multiply the given density by 1000 to get 1134 g/L.
Next, we can plug in the values we have into the formula:
Molarity = (1134 g/L) x (1/272.77 g/mol)
Molarity = 4.15 M
Therefore, the molarity of the 3.548 m solution of procaine hydrochloride is 4.15 M.
In explanation, molarity is a measure of the concentration of a solution, which is expressed in moles of solute per liter of solution. To calculate molarity, we need to know the mass of the solute in grams, the volume of the solution in liters, and the molecular weight of the solute in grams per mole. In this case, we were given the mass per volume (density) and the molecular weight, so we were able to convert the density to grams per liter and plug the values into the formula.
To learn more about Molarity refer to
https://brainly.com/question/2817451
#SPJ11
which of the following accurately describes the ph scale? which of the following accurately describes the ph scale? the ph scale runs from 0 (neutral) to 14 (most acidic), with 7 as an average acidity level. the ph scale runs from 0 (most acidic) to 14 (neutral), with 7 as an average acidity level. the ph scale runs from 0 (most basic) to 14 (most acidic), with 7 as a neutral. the ph scale runs from 0 (most acidic) to 14 (most basic), with 7 as a neutral.
Answers
Answer:
The pH scale measures acidity of a substance. known as potential of hydrogen, it varies from 0 to 14 with 7 being the pH value of a neutral solution. Below 7 shows the substance is acidic in nature and above 7 is alkaline in nature. pH 0-3 are considered strong acids while pH 4-6 are weak acids. pH 8-10 are weak alkalines and pH 11-14 are strong alkalines. This is a general trend and there may be exeptions especially if the substance has a negative pH. However, it would not be covered likely unless you are doing university chemistry.
the process that breaks down rocks
Answers
mercury thermometer should not be placed in the mouth of the children why?
Answers
Answer: If a pinch of mercury is consumed you will die from it so using a mercury thermometer is very unsafe if its breaks and a child consumes that mercury.
Explanation:
Don't use one
If the glass breaks and the mercury is not properly cleaned up, the little silvery ball within a mercury thermometer might be hazardous. As the mercury evaporates, it may pollute the air around and turn dangerous to both people and animals.
Children that have been exposed to mercury have lower IQs, hearing impairments, and worse coordination.
Long-term exposure worsens and exacerbates symptoms, which may lead to personality changes or even coma.
Mercury thermometers can be replaced by a number of things:
electronic thermometersGlass thermometers with gallium tinalcohol thermometers in glassThese non-mercury fever thermometers are significantly safer and equally accurate as mercury thermometers.
Read more about Mercury Thermometers :
https://brainly.com/question/27323100
PLEASE HELP!!!!!! SpongeBob Scientific Method Practice
Mr. Krabbs wants to make Bikini Bottoms a nicer place to live. He has created a
new sauce that he thinks will reduce the amount of bodily gas that comes out after
eating krabby patties from the Krusty Krab. He brings in 100 customers that
always have gas problems. He has 50 of them (Group A) eat krabby patties with
the new "Gas-free" sauce. The other 50 (Group B) eat krabby patties with sauce
that looks just like the Gas-free sauce but is really the old sauce with food
coloring. Both groups were told that they were getting Gas-free sauce. Two
hours after eating the krabby patties, 30 customers in Group A reported having fewer
gas problems and 2 customers in Group B reported having fewer gas problems.
I
1. What is Krusty Krab's hypothesis? Krusty Krab's hypothesis is that he thinks his
new sauce will reduce the amount of bodily gas that comes out after eatin
Answers
Answer:
The hypothesis is that the new sauce used in hamburgers will be able to reduce the incidence of body gases in customers.
Explanation:
A hypothesis is an assumption about a specific topic that is being researched. In the case shown in the question above, Krusty Krab believes that the new sauce he wants to use in hamburgers is capable of reducing the amount of body gases created by the body. Krusty Krab wanted to do scientific research where he analyzed the efficiency of this new sauce, but he assumes that the new sauce is efficient in reducing the creation of these gases. This is a hypothesis.
What are the types of nutrients that your body needs for energy? question 1 options: minerals vitamins macronutrients carbohydrates
Answers
Answer:
C. macronutrients
Explanation:
Macronutrients (carbohydrates, proteins, and fats) are needed for energy.
an aqueous solution of sodium sulfide has a concentration of 0.326 molal. the percent by mass of sodium sulfide in the solution is
Answers
The percent by mass of sodium sulfide in the solution is approximately 25.7%.
To calculate this, we use the equation: mass of solute / mass of solution x 100.
First, we need to calculate the mass of the sodium sulfide in the solution.
Since the concentration of the solution is given as 0.326 molal, we can calculate the mass using the following equation: mass = molal x molar mass.
The molar mass of sodium sulfide is 119.06 g/mol, so the mass of the sodium sulfide in the solution is 38.90 g.
Next, we need to calculate the total mass of the solution.
Since we are dealing with an aqueous solution, the mass of the water can be assumed to be the total mass of the solution.
The density of water is 1 g/mL, so we can calculate the total mass of the solution by multiplying the volume of the solution by the density.
Finally, we can use the equation: mass of solute / mass of solution x 100 to calculate the percent by mass of sodium sulfide in the solution. The result is approximately 25.7%.
In summary, the percent by mass of sodium sulfide in the solution is approximately 25.7%. This value was determined by calculating the mass of the sodium sulfide in the solution, the mass of the solution, and using the equation: mass of solute / mass of solution x 100.
To know more about percent by mass, refer here:
https://brainly.com/question/5394922#
#SPJ11
Which statement best describes what happens to the carbon dioxide produced by humans?.
Answers
NaBr (aq) + _H3PO4 (aq) → _Na3PO4(aq) + _HBr (aq)
3,1,1,3
1,2,3,1
3,1,3,1
1,1,3,3
Answers
Answer:
A 3,1,1,3
Explanation:
hope the picture help u to understand :)

What is Ksp for the following equilibrium if lithium phosphate has a molar solubility of 9.7×10−4 M?
Li3PO4(s)↽−−⇀3Li+(aq)+PO3−4(aq)?
Given that the Ksp value for AgNO2 is 8.5×10−4 M, if the concentration of Ag+in solution is 0.030 M, the concentration of NO−2 must exceed _____ mol/L to generate a precipitate.?
Answers
The Ksp value for Li3PO4 is 6.5 × 10^-25 M^4, calculated using the molar solubility of 9.7 × 10^-4 M.
The solubility product constant (Ksp) is a measure of the solubility of an ionic compound in water. It represents the equilibrium constant for the dissolution of a sparingly soluble ionic compound into its constituent ions in water. In this case, Li3PO4 is a sparingly soluble ionic compound, and its molar solubility is given. Using this solubility, we can calculate the Ksp value for the compound. In the second part of the question, the concentration of NO2- must exceed 2.8 × 10^-5 mol/L to generate a precipitate, calculated using the Ksp value of 8.5 × 10^-4 M and the solubility product expression of AgNO2. This calculation involves the use of the Ksp value and the principles of solubility equilibrium to predict the behavior of ionic solutions.
For more similar questions on topic "Solubility Equilibrium".
https://brainly.com/question/14409825
#SPJ11
Answer:
2.4E-11
Explanation:
For every equivalent of Li3PO4 that dissolves, three Li+ ions and one PO3−4 ion are released. If we abbreviate the given molar solubility of Li3PO4 as x (the amount of Li3PO4 that dissolves), we can relate this molar solubility to the expression for Ksp like so:
Ksp = [Li+]^3[PO3−4] = (3x)^3(x) = 27x^4
Substituting in the given value of x, 9.7E-4 M:
Ksp = 27 × [(9.7E−4)^4] = 2.4E−11
How many o atoms are in 259 g of c12h21o9? molar mass of c12h21o9 = 309.29 g/mol
Answers
The number of atoms in 259 g of C₁₂H₂₁O₉ is 45 x 10²³ oxygen.
What is molar mass?The molar mass of many compounds can be calculated by dividing the mass of the compound by the number of moles of the compound.
Given the molar mass is 309.29 g/mol
Given weight= 259g
number of moles = mass / molar mass
259g / 309.29g/mol =0.837mol
1 mol contains 6.022 x 10²³ molecules
0.837 mol contain 6.022 x 1023 x 0.837 = 5.0 x 10²³ molecules
1 molecule of C₁₂H₂₁O₉ contains 9 oxygen atoms
5.0 x 10²³ molecules of C₁₂H₂₁O₉ contain 9x 5 x 10²³ oxygen atoms.
=45 x 10²³ oxygen atoms
Thus, the number of atoms in 259 g of C₁₂H₂₁O₉ is 45 x 10²³ oxygen atoms
To learn more about molar mass, refer to the link:
https://brainly.com/question/22997914
#SPJ4
Answer:
Explanation: your the best
PLEASE ANSWER ASAP
A classmate argues that the changes in energy that occur in a pendulum are unrelated to the energy changes that a burning log undergoes. She points out that the burning of a log is an irreversible chemical change in matter, while the energy changes in a moving pendulum are constantly reversing, and are examples of physical changes. Evaluate your classmate’s argument.
Answers
Answer:
The argument about the chemical and physical changes in the energy is correct.
This is so because when a log is burnt, the chemical change occurs as wood releases energy in the form of carbon dioxide are formed which is irreversible while the changes in energy of movement of the pendulum is a physical change as its potential energy converts to kinetic and kinetic energy again converts to the potential which is a reversible process.
Hence, the argument saying the changes in energy occurring on a pendulum and log is right.
catalyst
A reaction between NH, and O, is the first step in the
preparation of nitric acid (HNO3) on a commercial scale.
The products are produced at 1000°C (1273 K) and at at-
mospheric pressure.
4 NH; (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (1)
a. What volume of NO is produced in the reaction vessel
by the reaction of 0.500 mol O2?
b. What mass of H2O is produced by the reaction of 15.0 L
of NH3?
c. How many liters of O, must react to produce 35.5 L of
NO?
Answers
a. volume of NO : 41.785 L
b. mass of H2O : 18 g
c. volume of O2 : 9.52 L
Further explanationGiven
Reaction
4 NH₃ (g) + 5 O2 (g) → 4 NO (g) + 6 H2O (l)
Required
a. volume of NO
b. mass of H2O
c. volume of O2
Solution
Assume reactants at STP(0 C, 1 atm)
Products at 1000 C (1273 K)and 1 atm
a. mol ratio NO : O2 from equation : 4 : 5, so mo NO :
\(\tt \dfrac{4}{5}\times 0.5=0.4\)
volume NO at 1273 K and 1 atm
\(\tt V=\dfrac{nRT}{P}=\dfrac{0.4\times 0.08206\times 1273}{1}=41.785~L\)
b. 15 L NH3 at STP ( 1mol = 22.4 L)
\(\tt \dfrac{15}{22.4}=0.67~mol\)
mol ratio NH3 : H2O from equation : 4 : 6, so mol H2O :
\(\tt \dfrac{6}{4}\times 0.67=1\)
mass H2O(MW = 18 g/mol) :
\(\tt mass=mol\times MW=1\times 18=18~g\)
c. mol NO at 1273 K and 1 atm :
\(\tt n=\dfrac{PV}{RT}=\dfrac{1\times 35.5}{0.08206\times 1273}=0.34\)
mol ratio of NO : O2 = 4 : 5, so mol O2 :
\(\tt \dfrac{5}{4}\times 0.34=0.425\)
Volume O2 at STP :
\(\tt 0.425\times 22.4=9.52~L\)
when melted iron solidifies without any change in temperature, what is happening on the atomic level? a. the iron atoms are gaining kinetic energy. b. the iron atoms are losing kinetic energy. c. the iron atoms are gaining potential energy. d. the iron atoms are losing potential energy.
Answers
The correct answer to the question is option B: the iron atoms are losing kinetic energy and potential energy when melted iron solidifies without any change in temperature.
When melted iron solidifies without any change in temperature, the iron atoms are losing kinetic energy, and they are losing potential energy as well.
During the process of melting, the iron atoms absorb energy, which makes them move more rapidly, and this increased kinetic energy enables them to overcome the intermolecular forces that hold them together in the solid state. As the temperature decreases, the kinetic energy of the iron atoms decreases, and eventually, they are no longer able to overcome these intermolecular forces. As a result, they begin to settle into a regular crystal lattice, and the iron solidifies.
At the same time, as the iron atoms settle into the crystal lattice, they release potential energy, which is stored in the bonds between the atoms. As the atoms become more tightly packed in the solid state, this potential energy is converted into kinetic energy, which causes the iron atoms to vibrate more slowly.
Learn more about potential energy here:
https://brainly.com/question/24284560
#SPJ11
A compound contains 36.48% Na, 25.41% S, and 38.11% O. Find it’s empirical formula
Answers
Answer:
we can use 100g of compounds as the basis.
Explanation:
Physical crowding and loud noise are considered sources of ___________ and are linked to the incidence of increased stress hormones.
Answers
Physical crowding and loud noise are considered sources of environmental stressors and are linked to the incidence of increased stress hormones.
High concentration of cytoskeletal filaments, organelles, and proteins along with the space constraints due to the axon’s narrow geometry lead inevitably to intracellular physical crowding along the axon of a neuron. . Molecular motors that mediate active transport share movement mechanisms that allow them to bypass physical crowding present on microtubule tracks. Everyday loud noise typically do not damage your hearing. However, many people participate in activities that produce harmful sound levels, which repeated over time will cause hearing loss.
learn more about:- Physical crowding & loud noise here
https://brainly.com/question/31149746
#SPJ11
1.
When 1• 50g of ethanol
was
apparatus 500g of water.
vapour
20°C to 39-5°C
enthalpy of compustion of
combustion
raised the temp forom
the
Calculate
ethanol
the
Answers
The heat that has been taken in by the water is 40.2 kJ.
What is the heat of combustion of the water?We know that when we heat the water the water would be turned from liquid to the vapor state . As such we are interested in the heat that would be evolved when this process would be taking place and we can be ale to write that;
H = mcdT
H = heat that have been evolved or absorbed
m = mass of the water
c = Heat capacity of the water
dT = The temperature change of the water.
As such, we have that;
H = 500 * 4.12 * (39.5 - 20)
H = 40.2 kJ
Learn more about enthlapy:https://brainly.com/question/13032919
#SPJ1
Match each image to the correct step of meiosis. FIRST WHO ANSWERS GETS BRAINLIEST!!
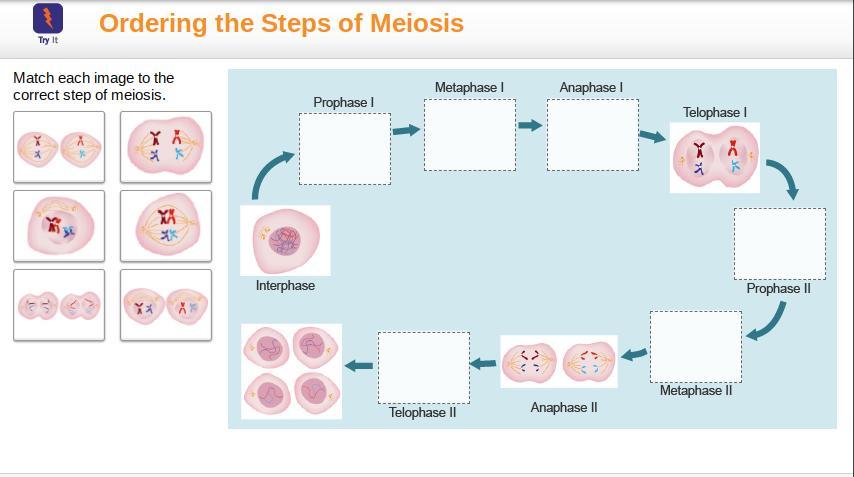
Answers
Answer:
505 908 0901 call my friend he has the answers
Explanation:
Why
isn't it possible to acheive 100% conversion in a series of CSTR
reactors?
Answers
It is not possible to achieve 100% conversion in a series of Continuous Stirred Tank Reactor (CSTR) due to various reasons.The following are the reasons why it is not possible to achieve 100% conversion in a series of CSTR reactors:1. Equilibrium Limitation:When a chemical reaction reaches equilibrium, the reaction rate becomes slow and constant. Equilibrium is a state in which the reactants and products are in equilibrium. When the concentration of reactants and products is equal, a chemical reaction is in equilibrium.
As a result, the reaction is not completely consumed and the conversion level is limited to less than 100%.2. Mass Transfer Limitation:Conversion in the CSTR is limited by the mass transfer between the phases, which is a limiting factor. Since the reactants must be brought into contact with each other to react, mass transfer is critical. As a result, there is a limit to the amount of reactant that can be converted.3. Residence Time:In a CSTR reactor, the product or reactant spends a certain amount of time. As a result, it is important to have the appropriate residence time for the reaction to occur. If the residence time is too short,
the reaction may not be completed, and if it is too long, the reaction may become too slow, resulting in a loss of product. This means that it is important to get the right balance between residence time and reaction rate to achieve a high level of conversion.4. Reactor Configuration:The reactor configuration also has an effect on the conversion level. Because of the CSTR's limited mixing, achieving high conversions necessitates the use of a reactor configuration that provides the highest possible degree of mixing. In a series of CSTRs, the conversion level is affected by how the reactors are connected. If the reactors are connected in parallel, the conversion level can be increased, but if they are connected in series, the conversion level will be reduced. As a result, the type of reactor used and the arrangement of reactors have an impact on the conversion level.
TO know more about that Equilibrium visit:
https://brainly.com/question/30694482
#SPJ11
The greater a habitat's biodiversity, the greater will be the habitat's, sustainability over time with varying conditions sustainability over time with varying conditions consumption of energy in the form of sunlight consumption of energy in the form of sunlight temperature ranges across the seasons temperature ranges across the seasons distance to the nearest water source distance to the nearest water source
Answers
Answer:
The correct answer is: sustainability over time with varying conditions.
Explanation:
A habitat can be defined as the physical and geographical conditions that will positively influence the development of life, in any form.
Therefore, it is correct to say that sustainability over time with favorable conditions will favor the greatness of the habitat.
Sustainability is the ability to preserve a system for the maintenance of future life, so it is ideal for there to be sustainable development across society, so that natural resources are preserved so that biodiversity continues to exist.
Help!
How do I find how many atoms of an element are in a sample?
Question 4 in picture

Answers
Answer:
D
Explanation:
The answer to question 4) is 5.79×10^22
Can someone explain me the energy transfer of a s’more in a campfire?
Answers
Answer:
Energy is the ability to do work, or in more simple terms: energy makes things happen. You use energy to ride your bike, play video games, bake cookies, and drive to school. Energy is exciting! Energy can be transferred from one object to another, and energy can be transferred into different forms, such as light, sound, and heat. When you sit by a campfire, you can feel the heat warm your body. The heat from the burning wood is transferred to your marshmallow, causing it to get soft and gooey. Perfect for your s’mores!
Heat can move from warm objects to cool objects, just like in the video when the heat from the wires made the paper ignite.
Explanation:
a sample contains 7.90 grams c and 42.1 grams s. you want to determine the empirical formula. how many moles of s are in the sample
Answers
Answer:
0.659
Explanation:
#brainliestisthekey
Answer: The answer is 1.31
Explanation:
a
In 25 words or fewer, what is the scientific question for the boiling
water experiment?
Answers
Answer: Does adding salt to water make the water boil faster that if salt is not added?
Explanation:
The scientific question for the boiling water experiment could be: "What is the effect of increasing temperature on the boiling point of water?"
This question aims to investigate how temperature influences the boiling point of water. By conducting the experiment and analyzing the results, scientists can gain a better understanding of the relationship between temperature and the physical state change of water from liquid to vapor.
The question focuses on a specific variable (temperature) and its impact on the boiling process, allowing for a targeted investigation and potential insights into the behavior of water under different conditions.
Know more about boiling point:
https://brainly.com/question/1514229
#SPJ2
what is the standard reduction potential, e, for the half-reaction al3+(aq) +
3e- + al(s)?
a. -0.76 v
b. 2.71 v
c. -1.68 v
d. 2.37 v
Answers
The standard reduction potential (e) for the half-reaction Al³⁺(aq) + 3e⁻ → Al(s) is -1.68 V.
The standard reduction potential (e) represents the tendency of a species to gain electrons and undergo reduction. It is measured in volts (V). To determine the standard reduction potential for the given half-reaction, we need to consult a table or reference that lists the standard reduction potentials.
The standard reduction potential for the reduction of Al³⁺(aq) to Al(s) can be found in standard electrochemical tables. The value for this half-reaction is -1.68 V. The negative sign indicates that the reduction process is spontaneous and favorable. It means that Al³⁺ ions have a higher tendency to gain electrons and form solid Al compared to the standard hydrogen electrode (which has a standard reduction potential of 0 V).
Therefore, the correct answer is option c: -1.68 V.
To learn more about electrons click here:
brainly.com/question/12001116
#SPJ11
