Answers
I would perfer to prefer to live my dream forever because I wouldn't let someone be stuck with my dream forever because I would rather tourture my self other than to toutour everybody else.
I would rather let everyone else live their dream, because living a dream forever after a certain point would get boring, no matter how fun it seems. Additionally, just because I sacrifice me having my dream doesn't mean I wouldn't be able to enjoy life. It just means that I wouldn't be able to live out my best scenario.
Related Questions
Write the formula of the conjugate acid of HCO₂⁻.
Answers
Taking into account the Brønsted-Lowry acid-base theory, the conjugate acid of HCO₂⁻ is H₂CO₂.
Brønsted-Lowry acid-baseThe Brønsted-Lowry acid-base theory (or the Brønsted-Lowry theory) identifies acids and bases based on whether the species accepts or donates protons or H⁺.
According to this theory, acids are proton donors while bases are proton acceptors. That is, an acid is a species that donates an H⁺ proton while a base is a chemical species that accepts an H⁺ proton from the acid.
So, reactions between acids and bases are H⁺ proton transfer reactions.
Conjugate base and conjugate acidThen, a conjugate base is an ion or molecule resulting from the acid that loses the proton, while a conjugate acid is an ion or molecule resulting from the base that gains the proton:
acid + base ⇄ conjugate base + conjugate acid
Conjugate acid of HCO₂⁻Like a conjugate acid is an ion or molecule resulting from the base that gains the proton, the conjugate acid of HCO₂⁻ is H₂CO₂.
Learn more about the Brønsted-Lowry acid-base theory:
brainly.com/question/12916250?referrer=searchResults
brainly.com/question/1191429?referrer=searchResults
brainly.com/question/4000152?referrer=searchResults
brainly.com/question/12808135?referrer=searchResults
Molecular compounds result from covalent bonding which are called _______.
This is for high school physical science
Answers
Answer:
Diatomic Molecule
Explanation:
Please answer this question


Answers
Answer:
I'm not an expert at this, but I assume its mercury.
A sample of an unknown compound is vaporized at 160 c . The gas produced has a volume of 2330 ml at a pressure of 1.00 atm ,and it weighs 2.10 g
Round answer to 3 significants digits

Answers
The molar mass is 3230.8 g/mol
How to determine the valueFirst, we need to know that the formula for the general gas law is represented as;
PV = nRT
such that the parameters are;
P is the pressureV is the volumen is the number of molesR is the gas constantT is the temperatureSubstitute the values
1 × 2.33 = n × 8.314 × 433.15
Multiply the values, we get;
n = 2.33/ 8.314 × 433.15
Divide the values
n = 6.5 × 10⁻⁴ moles
But, number of moles = mass/molar mass
Molar mass = 2.10/ 6.5 × 10⁻⁴
Molar mass = 3230.8 g/mol
Learn about ideal gas law at: https://brainly.com/question/25290815
#SPJ1
Seamus is conducting an experiment on electric force. He wants to get an approximate idea of how much force the charges will generate. Drag and drop the tiles to show the force of each situation in increasing order from lowest to highest (with repulsive forces being positive and attractive forces being negative).
=
One object with a charge of -4 × 10-5 C and another with a charge of 3 × 10-5 C placed 0.5
meters apart
One object with a charge of 3 x 10- C and another with a charge of -3 × 10-5 C placed 1
E
meter apart
= Two objects with a charge of 4 × 10-5 C placed 1 meter apart
= Two objects both with a charge of 3 × 10-5 C placed 0.5 meters apart
One object with a charge of 3 x 10- C and another with a charge of 4 x 10 C placed 1
E
meter apart

Answers
The highest electric force exerted by charges -4 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 0.5 m apart is equal to 43.15 N.
The lowest electric force exerted by charges 3 ×10⁻⁵ C and 3 ×10⁻⁵ C placed 1 m apart is equal to 8.10 N.
What is coulomb's law?According to Coulomb’s law, the force of attraction between two charges is equal to the product of their charges and is inversely proportional to the square of the distance. This electric force applies along the line joining the two charges.
The magnitude of the electric force can be written as follows:
\(\displaystyle F = k\frac{q_1q_2}{r^2}\)
where k is constant proportionality = 8.99 × 10⁹ N.m²/C².
Given the charge on one point charge, q₁ = 4 ×10⁻⁵ C
The charge on the other point charge, q₂ = - 3 × 10⁻⁵C
The distance between these two charges, r = 0.5 m
The magnitude of electric force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{4\times 10^{-5}\times 3\times 10^{-5}}{(0.5)^2}\)
F = 43.15 N
Given the charge on one point charge, q₁ = 3 ×10⁻⁵ C
The charge on the other point charge, q₂ = 3 × 10⁻⁵C
The distance between these two charges, r = 1 m
The magnitude of force between the charges will be:
\(\displaystyle F = 8.99\times 10^{9}\times \frac{3\times 10^{-5}\times 3\times 10^{-5}}{(1)^2}\)
F = 8.1 N
Learn more about Coulomb's law, here:
brainly.com/question/506926
#SPJ1
which function of lysosomes is carried out by vacuoles in plant cells
Answers
Answer:
Lysosomes are predominantly found in eukaryotic animal cells and are responsible for breaking down cellular debris
Explanation:
Classify each of these soluble solutes as a strong electrolyte, a weak electrolyte, or a nonelectrolyte. Drag each item to the appropriate bin. HCl, NaOH, HC2H3O2, HF, C2H5OH, HNO3, C6H12O6 Strong Electrolytes Weak Electrolytes Nonelectrolytes
Answers
Powerful Electrolytes:
HCl, NaOH, HNO3
Weak electrolyte:
HF, HC2H3O2
• Non-electrolytes:
C2H5OH, C6H12O6
Further explanation
An electrolyte solution is a substance that produces ions when dissolved in water and can conduct electricity.
Strong electrolytes are the solution
Solutes have the strongest electrical conductivity because they are completely ionized when dissolved in water.
• Weak electrolytes are partially ionized solutions with low electrical conductivity.
• Non-electrolytes are solutions that cannot conduct electricity because the solute cannot form ions. One of the most important properties of water is its ability to dissolve various substances. Solutions in which water is actually the dissolution medium are called aqueous solutions. Water is the most important solvent for electrolytes.
HCl = hydrochloric acid, a strong acid.
HNO3 = nitric acid, strong acid
• NaOH = sodium hydroxide, a strong base
HF = hydrofluoric acid, a weak acid
HC2H3O2 or CH3COOH = acetic acid, a weak acid
C2H5OH = ethanol, non-electrolyte
C6H12O6 = glucose, non-electrolyte
remarks:
• Some acids are fully ionized in water, while others are partially ionized. Not all acids are equally strong in generating H+ ions in solution. When an acid is fully ionized it is a strong acid. Click here when passing through hydrogen chloride
learn more about as a electrolyte ;
https://brainly.com/question/17089766
#SPJ4
For Hydrogen bonding H atom needs to be bonded to which other atoms?
Answers
Hydrogen bonding occurs when a hydrogen atom is covalently bonded to N, O, or F atom.
how many moles of HCL are required to prepare 0.80L of a 0.5M HCL solution
Answers
Answer:
\(0.4\text{ mol}\)Explanation:
Here, we want to get the number of moles
Mathematically:
\(Number\text{ of moles = molarity }\times\text{ volume}\)We have that as:
\(Number\text{ of moles = 0.5 }\times\text{ 0.8 = 0.4 mol}\)What is one difference between solids, liquids, and gases?
Answers
During a physics experiment, an electron is accelerated to 93 percent of the speed of light. What is the speed of the electron in miles per hour? (speed of light = 3.00 x 10^8 m/s, 1 km = 0.6214 mi)
Answers
speed of the electron = 0.93*speed of light
= 0.93*3.00 × \(10^{8}\) m/s*(0.6214 mi/1 km)*(3600 s/hr)
= 6.24X \(10^8\) mi/hr
What is speed?
Speed is defined as the rate at which the position of an object changes in any direction.
There are four types of speed and they are:
Constant Speed: An object is at a constant Speed when the object travels the same distance in equal intervals of time. Variable Speed: An object is said to have variable speed when the object travels varying distances at regular intervals. Average Speed: Average speed is defined as the constant speed obtained by dividing the total distance travelled by the object by the total time spent on the object. Instantaneous Speed : When an object moves with a variable speed, the speed of the object at any instant of time is called the instantaneous speed.To learn more about speed, visit;
https://brainly.com/question/13263542
#SPJ13
for a solution containing similar concentrations of hcn and nacn, . multiple choice if a small amount of acid is added, the hcn will neutralize it if a small amount of base is added, the ph would decrease very little if a small amount of acid is added, the ph would decrease very little if a small amount of base is added, the nacn will neutralize it if a small amount of acid is added, there would no change in ph
Answers
The pH would decrease very little if a small amount of acid is added.
What is pH value?The pH scale determines how acidic or basic water is. The range is 0 to 14, with 7 representing neutrality. Acidity is indicated by pH values below 7, whereas baseness is shown by pH values above 7. In reality, pH is a measurement of the proportion of free hydrogen and hydroxyl ions in water.
How does pH formula work?You need to know the hydronium ion concentration in moles per litre to determine the pH of an aqueous solution (molarity). The equation pH = - log [H3O+] is then used to determine the pH. Example: In a 0.0025 M HCl solution, determine the pH. Strong and entirely ionised in water, HCl is an acid.
Learn more about pH here:
brainly.com/question/15289741
#SPJ1
A student makes a saturated sugar solution by dissolving sugar grains in warm water stirring and adding more sugar until it no longer dissolves the student then allows the solution to come to room temperature the student pours equal volumes of sugar solution into five Beakers the student places a string into each of the beakers with one end of the string hanging free outside of the glassware the student then places the bigger the five chambers of varying temperatures 5,10,15,20 and 25°C for two weeks at the end of the experiment a student student noticed crystals have formed in the string the student masses the amount of crystals that formed from the sugar solution on the string select the dependent variable in this experiment what process in the rock cycle the student is most likely modeling
Answers
Answer:
The answer is A
Explanation:
A student who performed the Benedict tests on potatoes is the subject of a case study. Benedict Regent's flavor is the first thing that comes to mind. Benedict's solution is used to complete Benedict's test.
What sugars by dissolving sugar grains in warm water?With the aid of a potato, the kids tasted. To do the test, she utilized potato and Benedict solution. When Benedict solution is present, the color is a vivid crimson orange, however after the student's taste, complicated sugar does not.
There is no need to display a vivid orange or red color in Benedict's answer. The pupil was holding a potato. The brilliant orange color in the solution was displayed as a result. The two sugars' inclusion is what gives the mixture its hue.
An experiment should have two distinct controls. A positive control should be one, and a negative control should be the other.
Therefore, The one who oversees positive controls is called a positive control.
Learn more about sugar here:
https://brainly.com/question/18835784
#SPJ2
IF I try to dissolve 100 mg of substance X in 100 ml water at 90°C, what
will happen? What kind of solution will result?
Answers
If you try to dissolve 100 mg of substance X in 100 ml water at 90°C, the solubility of substance X will determine the type of solution that will result. Solubility is the maximum amount of a solute that can dissolve in a solvent at a specific temperature.
If substance X is soluble in water at 90°C, it will dissolve in the water to form a homogeneous solution. A homogeneous solution is a mixture of two or more substances that have a uniform composition and appearance. The dissolved substance X will be evenly distributed throughout the water, and the solution will be clear and transparent.
If substance X is not soluble in water at 90°C, it will not dissolve in the water, and a heterogeneous mixture will result. A heterogeneous mixture is a mixture of two or more substances that have a non-uniform composition and appearance. The undissolved substance X will remain as solid particles in the water, and the solution will be cloudy or turbid.
The solubility of substance X in water at 90°C can be influenced by several factors, including temperature, pressure, and the chemical nature of the solute and solvent. If you are unsure about the solubility of substance X in water at 90°C, you can consult reference tables or consult with a qualified chemist for advice.
For more such question substance
https://brainly.com/question/29108029
#SPJ11
_______-the phase where the
chromosomes pull apart
Answers
Answer:
The answer to your question is ⇔ anaphase
So it would be anaphase is the phase where the chromosones pull apart.
Explanation:
The sister chromatids are pairs of identical copies of DNA joined at a point called the centromere. During anaphase, each pair of chromosomes is separated into two identical, independent chromosomes. The chromosomes are separated by a structure called the mitotic spindle.
I hope this helps and have a wonderful day!
Hello what is the answer of question (a) please ?

Answers
Particle:
- Proton
Relative mass: 1
Relative charge: +1
Location in the atom: Nucleus
- Neutron:
Relative mass: 1
Relative charge: 0
Location in the atom: Nucleus
- Electron:
Relative mass: 1/1836
Relative charge: -1
Location in the atom: Electrosphere
Blue particle: proton
Black particle: neutron
Pink particle: electron
Yellow circle: electrosphere

Telluric acid (H2TeH4O6) is a diprotic acid with Ka1 = 2.0x10-8 and Ka2 = 1.0x10-11. A 0.25 M H2TeH4O6 contains enough HCl so that the pH is 3.00. What is the concentration of HTeH4O6
Answers
Answer:
5x10⁻⁶ = [HTeH₄O₆⁺]
Explanation:
The first dissociation equilibrium of the telluric acid in water is:
H₂TeH₄O₆ + H₂O ⇄ HTeH₄O₆⁺ + H₃O⁺
Using H-H equation for telluric acid:
pH = pKa + log₁₀ [HTeH₄O₆⁺] / [H₂TeH₄O₆]
pKa of telluric acid is -logKa1
pKa = -log 2.0x10⁻⁸
pKa = 7.699
As concentration of [H₂TeH₄O₆] is 0.25M, replacing in H-H equation:
3.00 = 7.699+ log₁₀ [HTeH₄O₆⁺] / [0.25M]
-4.699 = log₁₀ [HTeH₄O₆⁺] / [0.25M]
2x10⁻⁵ = [HTeH₄O₆⁺] / [0.25M]
5x10⁻⁶ = [HTeH₄O₆⁺]a solution of vinegar is what is the molality of acetic acid in vinegar which is 0.750 m in acetic acid, c2h4o2. the molar mass of acetic acid is 60.052 g/mol. the density of vinegar is 1.005 g/ml. group of answer choices
Answers
The molality of acetic acid in vinegar which is 0.750 m in acetic acid, C₂H₄O₂ is, 0.27M.
Molality is also known as molal concentration. It is a measure of solute concentration in a solution. The solution is composed of two components; solute and solvent.
Suppose the volume of solution is 1000 ml.
1.005 gm in 1 ml of solution.
1005 gm in 1000 ml of soln.
Number of moles = 0.750 M × 1 L = 0.750 mol
Mass of solute = n×M⋅wt = 0.750 × 60.052 = 45.039 gm
Mass of solvent = 1005 − 45.039 = 959.961 gm
Molality = \(\dfrac{45.039}{60.052} \times \dfrac{1000}{959.961}\)
= 0.27 M
To know more about the Molality, here
brainly.com/question/26921570
#SPJ4
What is the definition of specific heat?
OA. The total amount of energy contained within 1 mole of a
substance
OB. The heat required to break the molecular bonds within a
substance
C. The heat needed to raise the temperature of 1 g of a substance
1°C
D. The temperature change between the melting and boiling points of
a substance
Answers
Answer:
specific heat, the quantity of heat required to raise the temperature of one gram of a substance by one Celsius degree. The units of specific heat are usually calories or joules per gram per Celsius degrees.
Explanation:
the heat required to raise the temperature of the unit mass of a given substance by a given amount (usually one degree).
Which of the following is a possible
way to describe the SO3 component in
the reaction below?
Sa(s) + 120₂(g) → 8SO3(g)
A. 8 atoms SO3
B. 8 molecules SO3
C. 80.07g SO3
D. 32 LSO3
Answers
The correct answer is B. 8 molecules \(SO_3\). Option B
In the given reaction:
S(s) + \(O_2\)(g) → \(SO_3\)(g)
The stoichiometric coefficient in front of the \(SO_3\)molecule is 8, which indicates that 8 molecules of \(SO_3\)are formed as a product. This coefficient represents the ratio of the number of molecules involved in the reaction.
Option A (8 atoms \(SO_3\)) is incorrect because it only mentions the number of atoms, not molecules. The stoichiometric coefficient does not represent the number of atoms, but rather the number of molecules.
Option C (80.07g \(SO_3\)) is incorrect because it mentions a specific mass. The stoichiometric coefficient does not directly represent the mass of the substance, but rather the relative amount of molecules involved in the reaction.
Option D (32 \(SO_3\)) is incorrect because it mentions a specific volume. The stoichiometric coefficient does not directly represent the volume of the substance, but rather the relative amount of molecules involved in the reaction.
Therefore, the correct way to describe the \(SO_3\)component in the reaction is option B: 8 molecules \(SO_3\). This represents the ratio of the number of molecules of \(SO_3\)that are produced in the reaction.
Option B
For more wsuch question on molecules visit:
https://brainly.com/question/475709
#SPJ8
Which are examples of a phase change? (Select all that apply.)
evaporating water
cutting wood
frying eggs
melting butter
Answers
Answer:
melting butter and frying eggs
Explanation:
Hope this helps please mark me brainliest
volume reading
final: 28.5 mL
start: 7.5 mL
Total Volume: 21 mL
What is the Molarity of vinegar?
Based off the work information provided
Answers
The molarity of vinegar is 0.47368421 moles per liter.
To calculate this, we can use the following formula:
molarity = (initial_volume - total_volume_change) / final_volume
In this case, the initial volume is 7.5 mL, the total volume change is 21 mL, and the final volume is 28.5 mL. Plugging these values into the formula, we get:
molarity = (7.5 - 21) / 28.5 = -0.47368421
The negative value for molarity indicates that the solution is diluted. This is because the total volume of the solution increased by 21 mL, while the amount of solute (acetic acid) remained the same.
It is important to note that the molarity of a solution can change depending on the temperature. This is because the volume of a solution expands as it gets warmer. Therefore, it is important to measure the volume and temperature of a solution at the same time to get an accurate measurement of its molarity.
For such more questions on molarity
https://brainly.com/question/30704561
#SPJ8
the libretto of doctor atomic includes excerpts from the hindu bhagavad gita, even though oppenheimer was unfamiliar with that text. T/F
Answers
The libretto of doctor atomic includes excerpts from the Hindu Bhagavad gita, even though Oppenheimer was unfamiliar with that text. The statement is false.
What is the libretto of doctor atomic?The libretto of Doctor Atomic was created by Peter Sellars, drawing on original source material, including personal memoirs, recorded interviews, technical manuals of nuclear physics, declassified government documents, and the poetry of Muriel Rukeyser, an American poet and contemporary of Oppenheimer.
The storyline of Doctor atomic has it that scientists and soldiers were secretly stationed in Los Alamos, New Mexico, for the duration of World War II as they worked to bring a monstrous weapon to life.
Hence, Doctor Atomic focuses on the days and hours leading up to the first test of the bomb on July 16, 1945.
Learn more about Oppenheimer at: https://brainly.com/question/519587
#SPJ1
if two objects are in contact with each other and object A is at a lower temperature than object B, which of the following statements is correct?
a. the temperature of object A will decrease
b.the temperature of object B will decrease
c.both objects will remain the same temperature
d.heat energy will flow from object A to object B
Answers
A peak elutes from an HPLC column 17.7 cm in length in 11.1 min. What would be the width at half‑height of the peak (in seconds) if the plate height were 8.68 μm?
Answers
Answer:
10.98 s
Explanation:
To solve this problem we first use the formula:
H = L/NWhere:
H = Plate height (8.68 μm, or 8.68x10⁻⁶m)L = Column length (17.7 cm, or 0.177 m)N = Number of theoretical plates (unknown)And solve for N:
N = 0.177 m / 8.68x10⁻⁶mN = 20392 platesThen we use the formula:
N = 5.54*\((\frac{t_r}{W_{0.5}})^2\)Where:
N is the number of theoretical plates previously calculated. tr is the retention time (11.1 min, or 666s)W₀.₅ Is the width at half-height (unknown)And solve for W₀.₅:
W₀.₅ = 10.98 s24g copper is submerged in water in a graduated cylinder, the total volume increases bu 2.7ml what is the density of copper
Answers
Answer:
8.89 g/mLExplanation:
The density of a substance can be found by using the formula
\(density = \frac{mass}{volume} \\\)
From the question we have
\(density = \frac{24}{2.7} \\ = 8.88888...\)
We have the final answer as
8.89 g/mLHope this helps you
When zinc reacts with copper sulfate solution, zinc sulfate solution and copper are formed.(i) An experiment was carried out to measure the temperature change when zinc powder reactswith copper sulfate solution.initial temperature of copper sulfate solution = 20 °Cfinal temperature of mixture after the reaction = 46 °CExplain what the temperature readings show about the type of heat change that occurs duringthis reaction.
Answers
The temperature increase from 20 °C to 46 °C indicates that the reaction between zinc and copper sulfate solution is exothermic, with heat being released into the surroundings.
In the given reaction between zinc and copper sulfate solution, the temperature change can provide insights into the type of heat change occurring during the reaction. Based on the provided information, the initial temperature of the copper sulfate solution was 20 °C, and the final temperature of the mixture after the reaction was 46 °C.
The temperature increase observed in this reaction indicates an exothermic heat change. An exothermic reaction releases heat energy into the surroundings, resulting in a temperature rise. In this case, the reaction between zinc and copper sulfate solution is exothermic because the final temperature is higher than the initial temperature.
During the reaction, zinc displaces copper from copper sulfate to form zinc sulfate and copper metal. This displacement reaction is known as a single displacement or redox reaction. Zinc is more reactive than copper and therefore replaces copper in the compound.
The formation of new chemical bonds during the reaction releases energy in the form of heat. This energy is transferred to the surroundings, leading to an increase in temperature. The heat released is greater than the heat absorbed, resulting in a net increase in temperature.
The exothermic nature of this reaction can be explained by the difference in bond energies between the reactants and products. The breaking of bonds in the reactants requires energy input, while the formation of new bonds in the products releases energy.
In this case, the energy released during the formation of zinc sulfate and copper metal is greater than the energy required to break the bonds in copper sulfate and zinc.
For more such question on temperature visit:
https://brainly.com/question/4735135
#SPJ8
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
What does Newton’s first law of motion state?
Answers
Answer:
Newton's first law states that every object will remain at rest or in uniform motion in a straight line unless compelled to change its state by the action of an external force. This is normally taken as the definition of inertia. ... If that velocity is zero, then the object remains at rest.
Explanation:
Answer:
Before Galileo and Newton, many people thought that objects lost speed because they had a built-in natural tendency to do so. But those people weren't taking into account the multiple forces here on Earth - for example, friction, gravity, and air resistance - that cause objects to change their speed. If we could see the motion of an object in deep interstellar space, we would be able to observe the natural tendencies of an object that is free from any external influence. In deep interstellar space we would observe that if an object had a speed, it would continue to move with that speed until there was some force causing a change in its motion. Likewise, if an object were at rest in interstellar space, it would remain at rest until there was a force causing a change in its motion.
Explanation:
Hope it helped you =)
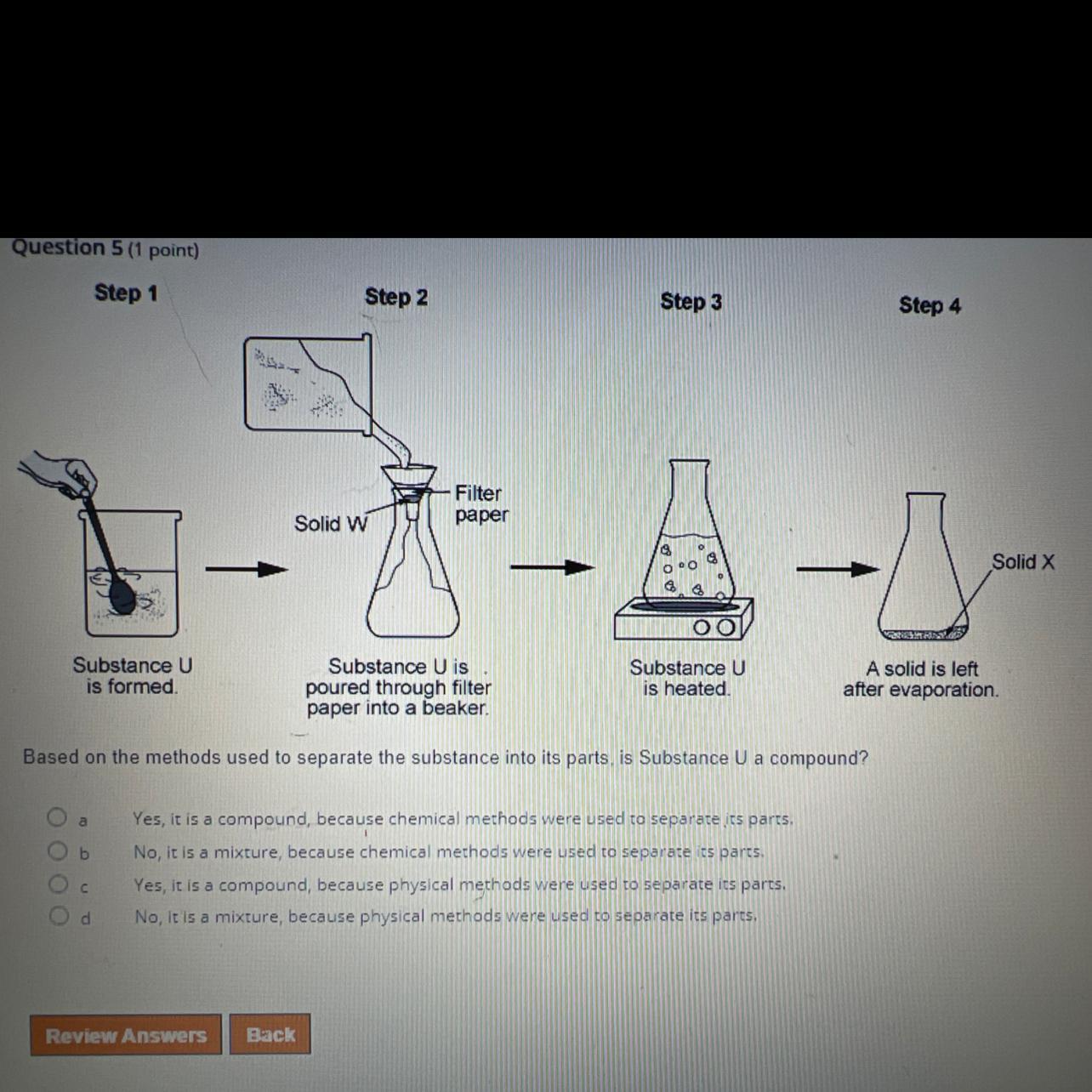
Based on the methods used to separate the substance into its parts is substance U a compound?
A. yes its a compound because chemical methods were used to separate its parts
B. no its a mixture because chemical methods were used to separate its parts
C. yes it is a compound because physical methods were used to separate its parts
D. no it is a mixture because physical methods were used to separate its parts
Answers
Based on the methods used to separate the substance into its parts is substance U is a mixture because physical methods were used to separate its parts; option D.
What are separation techniques?Separation techniques are techniques employed in the separation of mixtures of substances.
Mixtures are substance made up of two or more components physically joined together.
Considering the substance which is being separated, the separation techniques employed are physical separation techniques, hence the substance is a mixture.
In conclusion, physical separation techniques are employed in the separation of mixtures.
Learn more about separation techniques of mixtures at: https://brainly.com/question/4825542
#SPJ1