Answers
Answer:
Net ionic equation is;
(Ag+)(aq) + (F-)(aq) = (AgF) (s)
Explanation:
Silver acetate has a molecular formula of CH_3 - COO - Ag
Potassium fluoride has a molecular formula of KF.
Thus, molecular reaction is;
CH_3 - COO - Ag + KF = CH_3 - COO - K + AgF
Now we are told that they are both aqueous solution. This means that only the aqueous phase is ionized.
Thus, the total ionic equation is;
((CH_3 - COO)-)(aq) + (Ag+)(aq) + (K+)(aq) + (F-)(aq) = ((CH_3 - COO)-)(aq) + (K+)(aq) + (AgF) (s)
The spectator ions in the total net equation are;((CH_3 - COO)-) and (K+). Thus, they are not going to appear in the net ionic equation.
Net ionic equation is;
(Ag+)(aq) + (F-)(aq) = (AgF) (s)
Related Questions
what products are formed if the compound is allowed to sit in d2O that contains a small amount of D2SO4
Answers
Answer:
the products of the equations is D₃O⁺ + DSO₄⁻
Explanation:
attached is the diagram which explain the reaction
reaction of the equation
D₂O + D₂SO₄ ----------- D₃O⁺ + DSO₄⁻
Note: in phenol, OH is in an ortho and para direction
ortho and para direction indicates direction of non-hydrogen substitute on hydrocarbon ring(benzene family)

1 gallon =3.785 L how many liters of gasoline will fill a 10.00 tank
Answers
Answer:
37.85 L
Explanation:
3.785 x 10.00 = 37.85 L
it would take 37.85 L to fill a 10.00 tank
(sorry if im wrong pls dont report)
(hope this helps can i plz have brainlist :D hehe)
Describe the unique properties and biological importance of the two important groups of macromolecules in your life and briefly explain their functions in the body. Remember that you sp KB BBC x tre Discuss the structural components of these molecules and explain the process that joins these molecules in polymersq. B omm.
Answers
Explanation
2 important groups of macromolecules:
Carbohydrates
These substances are an essential part of our diet (for example grains, fruits, and vegetables). They provide energy to the body, particularly through glucose, a simple sugar that is a component of starch and an ingredient in many staple foods.
Carbs also have other important functions in humans, animals, and plants.
Proteins
These are one of the most abundant organic molecules in living systems and have the most diverse range of functions of all macromolecules. Proteins may be structural, regulatory, contractile, or protective; they may serve in transport, storage, or membranes; or they may be toxins or enzymes. Each cell in a living system may contain thousands of proteins, each with a unique function. Their structure, like their function, varies greatly.
A solution contains 3.5 mol NaCl and 4.2 mol MgCl₂. How many equivalents of chloride ion are present?
Answers
There are 15.4 equivalents of chloride ion present in the solution
To calculate the number of equivalents per mole of chloride ionWe need to multiply the total number of moles of chloride ion in the solution by the number of equivalents.
The molar mass of NaCl is 58.44 g/mol, so 3.5 mol of NaCl contains :
3.5 mol NaCl x 2 mol Cl⁻/1 mol NaCl = 7 mol Cl⁻
Similarly, the molar mass of MgCl₂ is 95.21 g/mol, so 4.2 mol of MgCl₂ contains:
4.2 mol MgCl₂ x 2 mol Cl⁻/1 mol MgCl₂ = 8.4 mol Cl⁻
Therefore, the total number of moles of chloride ion in the solution is:
7 mol Cl⁻ + 8.4 mol Cl⁻ = 15.4 mol Cl⁻
By dividing the total number of moles by the number of equivalents per mole, we can finally determine how many equivalents of the chloride ion there are. There is one equivalent of the chloride ion per mole since it has a valency of -1.
15.4 mol Cl⁻ x 1 eq/mol = 15.4 eq
So there are 15.4 equivalents of chloride ion present in the solution.
Learn more about mole here : brainly.com/question/30337257
#SPJ1
.
Ag+1, NO3-1
+
Na+1, I-1
->
Answers
Answer:
0.3+Na
Explanation:
Liquid octane (CH3(CH2)6CH3) will react with gaseous oxygen (O2) to produce gaseous carbon dioxide (CO2) and gaseous water (H2O). Suppose 17. g of octane is mixed with 112. g of oxygen. Calculate the maximum mass of water that could be produced by the chemical reaction. Round your answer to significant digits.
Answers
The maximum mass of water that could be produced by the chemical reaction is 162 g.
The given chemical equation is: 2 C8H18(l) + 25 O2(g) → 16 CO2(g) + 18 H2O(l)In the chemical reaction of liquid octane with gaseous oxygen, the products are gaseous carbon dioxide and gaseous water.According to the balanced chemical equation, 2 moles of C8H18 react with 25 moles of O2 to form 18 moles of H2O.So, 1 mole of C8H18 react with 25/2 = 12.5 moles of O2 to form 9 moles of H2O.The molar mass of C8H18 is 114 g/mol. So, the number of moles in 17 g of C8H18 is:17 g / 114 g/mol = 0.149 molThe molar mass of O2 is 32 g/mol. So, the number of moles in 112 g of O2 is:112 g / 32 g/mol = 3.5 molFrom the balanced chemical equation, 1 mole of C8H18 react with 12.5 moles of O2 to form 9 moles of H2O.So, the number of moles of O2 required to react with 0.149 mol of C8H18 to form H2O is:(12.5 mol / 1 mol) × (0.149 mol / 2 mol) = 0.935 molThe maximum number of moles of H2O that can be produced from 0.149 mol of C8H18 and 0.935 mol of O2 is 9 mol.So, the mass of water produced from 17 g of C8H18 and 112 g of O2 is:9 mol × 18 g/mol = 162 g
for more questions on chemical reaction
https://brainly.com/question/25769000
#SPJ8
What volume of a 2.46 M
magnesium nitrate (Mg(NO3)2)
solution would be needed to make
275 mL of a 0.758 M solution by
dilution?
[?] mL of 2.46 M Mg(NO3)2
Volume (mL)
Enter
Help Reso
Answers
The volume of 2.46 M magnesium nitrate solution needed to make 275 mL of 0.758 M solution by dilution would be 84.6 mL.
Dilution problemTo solve this problem, we can use the dilution formula, which is:
M1V1 = M2V2
where M1 is the initial concentration, V1 is the initial volume, M2 is the final concentration, and V2 is the final volume.
We can rearrange this equation to solve for the initial volume, V1:
V1 = (M2V2) / M1
Plugging in the given values, we get:
M1 = 2.46 M
M2 = 0.758 M
V2 = 275 mL = 0.275 L
V1 = (0.758 M)(0.275 L) / 2.46 M
V1 = 0.0846 L or 84.6 mL
Therefore, we need 84.6 mL of the 2.46 M magnesium nitrate solution to make 275 mL of a 0.758 M solution by dilution.
More on dilution can be found here: https://brainly.com/question/28997625
#SPJ1
Which of the following is an incorrect representation for a neutral atom?
36Li
613C
3063Cu
1530P
Answers
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97. The incorrect representation for a neutral atom is 36Li
To determine the correct representation for a neutral atom, we need to consider the atomic number (Z) and mass number (A) of the element. The atomic number represents the number of protons in the nucleus, while the mass number represents the sum of protons and neutrons.
Let's analyze the given representations:
36Li:
This representation suggests that the element is lithium (Li) with a mass number of 36, which is incorrect. The correct mass number for lithium is approximately 6.94.
613C:
This representation suggests that the element is carbon (C) with a mass number of 13, which is correct. Carbon has different isotopes, and 13C represents one of its stable isotopes.
3063Cu:
This representation suggests that the element is copper (Cu) with a mass number of 63, which is correct. Copper has different isotopes, and 63Cu represents one of its stable isotopes.
1530P:
This representation suggests that the element is phosphorus (P) with a mass number of 30, which is incorrect. The correct mass number for phosphorus is approximately 30.97.
Therefore, the incorrect representation for a neutral atom is 36Li, as it does not match the known properties of lithium.
For more question on atom
https://brainly.com/question/26952570
#SPJ8
A construction company is building a house. After a truck delivers the materials, workers build a wooden frame, cover
the exterior with bricks, and spread gravel for the driveway.
Which resource used in the scenario is nonrenewable?
the wooden frame of the house
the gravel rocks in the driveway
the diesel fuel in the delivery truck
the clay bricks on the outside of the houses
Answers
Answer:
The diesel fuel in the delivery truck
Explanation:
Answer:
The answer is c
Explanation:
The diesels fuel in the delivery truck
Question 1
1 pts
2B+6HCI --
| --> 2BCl3 + 3H2
How many moles of boron chloride will be produced if you start with 8.752 moles of HCI
(hydrochloric acid)? (Round to 3 sig figs. Enter the number only do not include units.)
Answers
Answer:
2.92 mol
Explanation:
Step 1: Write the balanced equation
2 B(s) + 6 HCI(aq) ⇒ 2 BCl₃(aq) + 3 H₂(g)
Step 2: Establish the appropriate molar ratio
The molar ratio of hydrochloric acid to boron chloride is 6:2.
Step 3: Calculate the moles of boron chloride produced from 8.752 moles of hydrochloric acid
\(8.752molHCl \times \frac{2molBCl_3}{6molHCl} = 2.92molBCl_3\)
arrange the sub levels in ascending order by the number of electrons they can obtain
Answers
The sub-levels in order of the number of electrons they can hold will be s, p, d, and f.
What are sub-levels?There are 4 sub-levels, namely; s, p, d, and f.
Each level represents an orbital with some orbitals having multiple degenerates.
s orbital can take a maximum of 2 electronsp orbitals can take a maximum of 6 electronsd orbitals can take a maximum of 10 electronsf orbital can take a maximum of 14 electrons.Thus, the ascending order in terms of the number of electrons each sublevel can hold will be s, p, d, and f.
More on orbitals can be found here: https://brainly.com/question/18914648
#SPJ1
Explain what the biosphere is
Answers
Answer:
Explanation:
Natural vegetation and wildlife exist only in the narrow zone of contact between the lithosphere, hydrosphere and atmosphere that we call biosphere. In the biosphere living beings are inter-related and interdependent on each other for survival. This life supporting system is known as the ecosystem.
Hope this helps
Plz mark as brainliest!!!!!!!!!
What is the rate constant of a first-order reaction when 20.0% of a reactant remains after 73.5 s?
Answers
Answer:
Half-lives of first order reactions
Explanation:
Notice the the half-life is independent of initial concentration. This is not the case with other reaction orders. The half-life of a first-order reaction was found to be 10 min at a certain temperature. hope this helps you :)
0.0453 sec⁻¹ the rate constant of a first-order reaction when 20.0% of a reactant remains after 73.5 s.
What is rate constant?The constant is indeed a proportional factor in chemical kinetics' rate equation that connects reactant molar concentration to reaction rate. It is additionally known as the pre - exponential factor or reaction rate factor and is represented by the symbol k in an equation.
The constant, k, is indeed a constant of proportionality that illustrates the connection between reactant molar concentration and reaction rate. Using the molar quantities of the reagents and the sequence of reaction, the constant may be determined experimentally. It may also be computed using the Following equations.
t1/2 = 0.693/k
Fraction remaining = 0.5n
n = number of half lives
0.20 = 0.5n
log 0.20 = n log 0.5
n = 2.32
2.32 half lives = 35.5 sec
1 half life = 15.3 sec
15.3 sec = 0.693/k
k = 0.0453 sec-1
Therefore, 0.0453 sec⁻¹ the rate constant of a first-order reaction when 20.0% of a reactant remains after 73.5 s.
To know more about rate constant, here:
https://brainly.com/question/14977272
#SPJ6
Each of the following processes caused the gas volume to double, as shown. For each process, tell how the remaining gas variable changed or state that it remained fixed: A. T doubles at fixed P. B. Tand n are fixed C. At fixed T, the reaction is CD2(g) → C(g) + D2 (g) D. At fixed P, the reaction is Az(g) + B2(g) → 2 AB (g)
Answers
Answer: (A) V doubles, (B) P decreases by double, (C) n increases by double, (D) T increases by double
Explanation:
With the given information, we are looking at a problem that is related to the ideal gas law: PV = nRT, where
P = pressureV = volumen = amount of the gas, in molesR = ideal gas constant (value and units vary depending on the units of the other terms)T = temperatureFor this problem, we do not need to know any discrete values. Rather, we can use PV = nRT as a guiding equation to predict changes if any of the variables are changed. We are told the gas volume doubles in each of the following processes.
A. If T doubles, while P remains fixed, that means in order for the PV term to remain proportional to T in the ideal gas equation, V has to double since P is fixed. We can assume n and R are not changing in each of the following processes since these properties are inherent to the gas molecules present, leaving the relationship as PV ∝ T
B. If T and n are fixed as V doubles, this means P will decrease by double as well. Since R is a constant, that leaves the following relationship PV = K, where K represents some constant value comprised of the product of T, n, and R. Therefore, P and V are inversely proportional to each other (P ∝ 1/V).
C. At fixed T and given the reaction CD2(g) → C(g) + D2 (g), we see that the number of moles in gas molecules (n) increases via a decomposition reaction. Therefore, n doubles as V doubles.
D. At fixed P and given the reaction Az(g) + B2(g) → 2 AB(g), we see that a combination reaction occurs but the number of moles of the gas stays the same. This is because there is.1 mole of Az and 1 mole of B2 as reactants, compared to 2 moles of AB product. Thus, we started with 2 moles as reactants and ended with 2 moles of product. Since we are told that V doubles, then T increases by double to maintain the ideal gas laws.
Is the cell wall factory part or worker? And please explain what the worker or factory part does. (Don’t tell me it’s on quizlet bc I can’t find it)
Answers
A gas mixture (40% CH4, 40% CO, and 20% H2) are burned with 300% excess air where the gas and air
entering the combustion chamber at 25 C. What is the theoretical flame temperature achieved in C
Answers
pOH of the 0.001M NaOH solution is
Answers
The pOH of the 0.001 M NaOH solution is approximately 3.
To determine the pOH of a solution, we need to know the concentration of hydroxide ions (OH-) in the solution.
In the case of a 0.001 M NaOH solution, we can assume that all of the NaOH dissociates completely in water to form Na+ and OH- ions. Therefore, the concentration of hydroxide ions in the solution is also 0.001 M.
The pOH is calculated using the equation:
pOH = -log[OH-]
Substituting the concentration of hydroxide ions, we have:
pOH = -log(0.001)
Using a calculator, we can evaluate the logarithm:
pOH ≈ 3
Therefore, the pOH of the 0.001 M NaOH solution is approximately 3.
Know more about hydroxide ions here:
https://brainly.com/question/28464162
#SPJ8
Which front forms widespread clouds, rain, or snow?
Answers
Answer:
Warm front
Explanation:
Warm front forms widespread clouds, rain, or snow.
Answer:
Warm front
Explanation:
Just took the quiz
Starting with 2.50 mol of N2 gas (assumed to be ideal) in a cylinder at 1.00 atm and 20.0C, a chemist first heats the gas at constant volume, adding 1.36 * 104 J of heat, then continues heating and allows the gas to expand at constant pressure to twice its original volume. Calculate (a) the final temperature of the gas; (b) the amount of work done by the gas; (c) the amount of heat added to the gas while it was expanding; (d) the change in internal energy of the gas for the whole process.
Answers
Answer:
a) \(T_b=590.775k\)
b) \(W_t=1.08*10^4J\)
d) \(Q=3.778*10^4J\)
d) \(\triangle V=4.058*10^4J\)
Explanation:
From the question we are told that:
Moles of N2 \(n=2.50\)
Atmospheric pressure \(P=100atm\)
Temperature \(t=20 \textdegree C\)
\(t = 20+273\)
\(t = 293k\)
Initial heat \(Q=1.36 * 10^4 J\)
a)
Generally the equation for change in temperature is mathematically given by
\(\triangle T=\frac{Q}{N*C_v}\)
Where
\(C_v=Heat\ Capacity \approx 20.76 J/mol/K\)
\(T_b-T_a=\frac{1.36 * 10^4 J}{2.5*20.76 }\)
\(T_b-293k=297.775\)
\(T_b=590.775k\)
b)
Generally the equation for ideal gas is mathematically given by
\(PV=nRT\)
For v double
\(T_c=2*590.775k\)
\(T_c=1181.55k\)
Therefore
\(PV=Wbc\)
\(Wbc=(2.20)(8.314)(1181_590.778)\)
\(Wbc=10805.7J\)
Total Work-done \(W_t\)
\(W_t=Wab+Wbc\)
\(W_t=0+1.08*10^4\)
\(W_t=1.08*10^4J\)
c)
Generally the equation for amount of heat added is mathematically given by
\(Q=nC_p\triangle T\)
\(Q=2.20*2907*(1181.55-590.775)\\\)
\(Q=3.778*10^4J\)
d)
Generally the equation for change in internal energy of the gas is mathematically given by
\(\triangle V=nC_v \triangle T\)
\(\triangle V=2.20*20.76*(1181.55-293)k\)
\(\triangle V=4.058*10^4J\)
Some organic solvents do not work well in liquid-liquid aqueous extractions. Ethanol (HOCH2CH3) is a common inexpensive solvent, but is a poor solvent for extractions. In ten or fewer words, provide an explanation for why ethanol is a poor solvent selection for extraction.
Answers
Answer:
See explanation
Explanation:
Extraction has to do with the separation of the components of a mixture by dissolving the mixture in a set up involving two phases. One phase is the aqueous phase (beneath) while the other is the organic phase (on top). The solvents used for the two phases must not be miscible. Water commonly is used for the aqueous phase.
Ethanol is an important solvent in chemistry but the solvent is miscible with water in all proportions. As a result of this, ethanol is a poor solvent for carrying out extraction.
Jessa examines a bullet at a crime scene. The markings, called
Answers
1) She would find "ballistic markings" or "striations."
2) She would find the whorl pattern of finger prints
What are the markings called?As a crime scene investigator, Jessa will search for distinctive signs on the bullet that could identify it as coming from a particular firearm. Common names for these stains include "ballistic markings" and "striations." When a bullet travels through a gun's barrel, it may leave behind distinctive traces due to flaws in or wear on the rifling, spiral grooves carved into the barrel to stabilize the projectile. These traces are imprinted on the bullet's surface and can be examined to identify the weapon type.
Jessa will likely use a comparison microscope to inspect the bullet and compare the ballistic marks to test-fired bullets from potential guns.
Learn more about bullet:https://brainly.com/question/14290893
#SPJ1
Missing parts;
Jessa is a crime scene Investigator. She examines a bullet found at the scene and a fingerprint left in a blood smear. What type of marks might
link the bullet to a specific weapon? Which type of fingerprints has she found?
Jessa examines a bullet at a crime scene. The markings, called might link the bullet to a specific weapon. She also finds some
fingerprints smeared in the blood of the victim. These are
fingerprints.
PLEASE ANSWER SOON AS POSSIBLE
What is the volume, in liters, of 1.20 mol of C3H8 gas at STP
Answers
26.8L is the volume, in liters, of 1.20 mol of C\(_3\)H\(_8\) gas at STP. A measurement of three-dimensional space is volume.
A measurement of three-dimensional space is volume. It is frequently expressed quantitatively using SI-derived units, like the cubic metre or litre, or different imperial or US-standard units, including the gallon, quart and cubic inch. Volume and length (cubed) have a symbiotic relationship.
The volume of an envelope is typically thought of as its capacity, not as the amount of space it takes up. In other words, the volume is the quantity of fluid (liquid or gas) that the container may hold.
Volume = 1.20×22.4
=26.8L
To know more about volume, here:
https://brainly.com/question/1578538
#SPJ1
An aqueous solution ggggggggggggg
Answers
Answer:
cool
Explanation:
the density of lead is 11.342 g/ml. what would be the volume of a 25.00 g sample of this metal?
Answers
The volume of the lead is 2.2 mL
What is density?The term density refers to the ratio of the mass to the volume of a body. We know that from the question, the following are the parameters that we have been given;
Density of lead = 11.342 g/ml
Mass of lead = 25.00 g
Volume of lead = ??
We can write that;
Density = mass/volume
Volume = mass/Density
Volume = 25.00 g/ 11.342 g/ml
= 2.2 mL
Learn more about density:https://brainly.com/question/15164682
#SPJ1
A container holds 6.4 moles of gas. Hydrogen gas makes up 25% of the total moles in the container. If the
total pressure is 1.24 atm. What is the partial pressure of hydrogen? Must show work! Use P^a/P^t = n^a/n^t
Answers
Answer:
i let alone need more time to figure this out and i will get you the awnser.
Explanation:
write the products that form for the following reaction Al + Ca(NO3)2
Answers
The following balanced chemical equation may be used to describe the interaction between aluminum (Al) and calcium nitrate (Ca(NO₃)₂):
2 Al + 3 Ca(NO₃)₂ → 2 Al(NO₃)3 + 3 Ca
Reactants are the chemicals that begin a chemical reaction, while products are the compounds that are created as a result of the reaction.
The substances that initiate a chemical reaction. Products are the substances that are created during the reaction. Compounds or elements can act as reactants and products.
Aluminium and calcium nitrate interact in this reaction to form aluminium nitrate (Al(NO₃)₃) and calcium (Ca), which are the end products.
Learn more about chemical equation, here:
https://brainly.com/question/28972826
#SPJ1
During chemical reaction 7.55gKI and 9.06g were allowed to react. How many grams of excess reagent are left over after the reaction is complete. Reaction: Pb(NO3)2(s) + 2KCI(s) > 2KNO3(s) + PbI(s)
Answers
Answer: 7.45 g of \(Pb(NO_3)_2\) excess reagent are left over after the reaction is complete.
Explanation:
To calculate the number of moles, we use the equation:
\(\text{Number of moles}=\frac{\text{Given mass}}{\text{Molar mass}}\)
a) \({\text{Number of moles of} KI}=\frac{7.55g}{166g/mol}=0.045moles\)
b) \({\text{Number of moles of} Pb(NO_3)_2}=\frac{9.06g}{331.2g/mol}=0.027moles\)
The balanced chemical reaction is :
\(Pb(NO_3)_2(s)+2KI(s)\rightarrow 2KNO_3(s)+PbI(s)\)
According to stoichiometry :
2 moles of \(KI\) require = 1 mole of \(Pb(NO_3)_2\)
Thus 0.045 moles of \(KI\) will require=\(\frac{1}{2}\times 0.045=0.0225moles\) of \(Pb(NO_3)_2\)
Thus \(KI\) is the limiting reagent as it limits the formation of product and \(Pb(NO_3)_2\) is the excess reagent as (0.045-0.0225) = 0.0225 moles are left
Mass of \(Pb(NO_3)_2=moles\times {\text {Molar mass}}=0.0225moles\times 331.2g/mol=7.45g\)
Thus 7.45 g of \(Pb(NO_3)_2\) of excess reagent are left over after the reaction is complete.
A hit-and-run accident has left a pedestrian injured. The investigators have used eyewitness testimony and motor vehicle records to narrow down the list of suspects. When one of these suspects is interviewed, he is asked if they would find any blood on his car. He says that he hit a deer a few weeks ago, so it is possible that there is blood. How could this statement be tested?
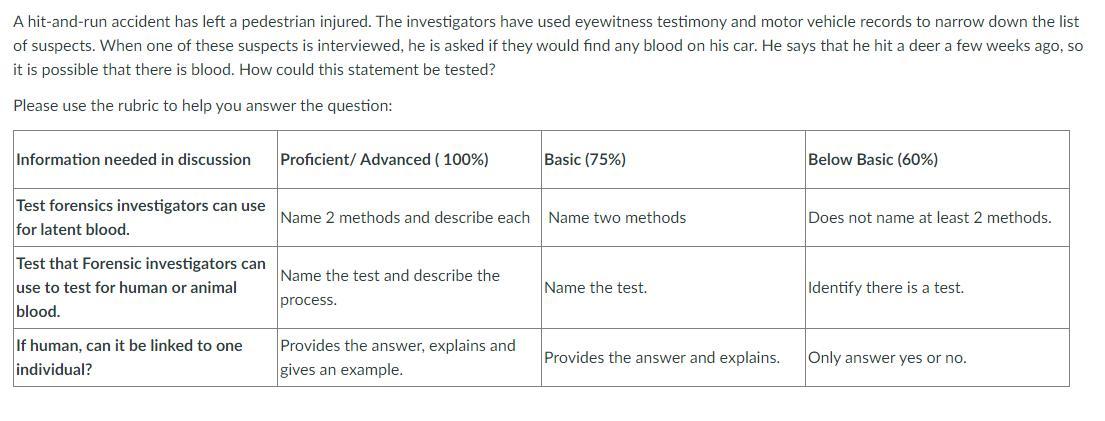
Answers
Test forensic investigators can use to test for human or animal blood will be
required to test the statement.
What is Forensics?These are different tests and techniques used by appropriate body to
investigate a crime in order to know what really happened and who is
responsible.
The test which can be used to differentiate between human and animal
blood is Precipitin Test. This is done by identifying the presence of proteins
that are found only in human blood such as albumin.
Read more about Blood tests here https://brainly.com/question/3731326
20 POINTS PLEASE ANSWER
In the following reaction, if you wanted to produce more hydrochloric acid (HCl), what should you do? (2 points)
4HCl + O2 ⇄ 2H2O + Cl2
Select one:
a. Add more H2O
b. Add more O2
c. Remove H2O
d. Remove Cl2
Answers
Answer:
Add more H2O
Explanation:
Thomas wants to measure the temperature change that occurs when sodium hydroxide is dissolved in water.
Which is the best tool for this purpose?
Answers
Answer: A thermometer
Explanation: The best tool for measuring temperature change in this scenario would be a thermometer. A thermometer is specifically designed to measure temperature and can accurately indicate changes in temperature when substances are mixed or undergo reactions. When sodium hydroxide (NaOH) is dissolved in water, it is an exothermic reaction, meaning it releases heat. By using a thermometer, Thomas can monitor and measure the temperature change that occurs as sodium hydroxide dissolves in water. It is important to select a suitable thermometer that can accurately measure the desired temperature range and ensure that it is calibrated correctly for accurate readings. Additionally, it is advisable to use a thermometer that is specifically designed for liquid measurements, such as a liquid-in-glass thermometer or a digital thermometer with a suitable probe for liquid measurements. These types of thermometers provide reliable temperature readings and are commonly used in scientific experiments and laboratory settings to measure temperature changes accurately.