Write the symbols for the ions that form when
potassium and iodine react to form the ionic compound potassium
iodide. for middle school
Answers
Answer:A sodium atom transfers an electron to a chlorine atom to form a sodium ion and a chloride ion. The product is the ionic compound, sodium chloride. In the same way, a potassium atom transfers an electron to an iodine atom to form a potassium ion and an iodide ion. So, potassium iodide is an ionic compound.
Explanation:
Related Questions
chemicals x and y (both gases) react to form the gas xy, but it takes a bit of time for the reaction to occur. both x and y are placed in a container with a piston (free to move), and you note the volume. as the reaction occurs, what happens to the volume of the container? explain.
Answers
As the reaction between the two gases proceeds, the volume of the container will decrease due to the formation of a new gas, XY, which takes up less space than the two gases combined.
In a closed system, the decrease in volume is accompanied by an increase in pressure, as the molecules of XY have less space to move around in than the molecules of X and Y.
This is because the increased pressure will cause the molecules to move faster, thereby increasing the kinetic energy of the system. This increase in temperature will cause the reaction to speed up, leading to more molecules of XY being formed. As the reaction continues, the pressure, temperature, and volume will continue to change in a dynamic equilibrium until all the reactants are used up.
Learn more about gases:
https://brainly.com/question/27660102
#SPJ4
what type of energy warms earths surface?
A. Solar energy
B. Gravitational energy
C. Nuclear energy
D. Thermal energy
Answers
a. solar energy
the earth is warmed by the sun, which is solar (or infrared).
Which mass of magnesium sulphate will be formed if 12 g of magnesium are reacted with sulphuric acid?
Answers
Answer:
magnesium hydrosulphate
A student dissolves a Jefferson nickel to make 100.00 mL of solution in a volumetric flask. The student takes a 5.00 mL aliquot of the first solution and dilutes it to make 100.00 mL of a second solution. The student places a sample of the second solution in a cuvette for analysis using spectrophotometry.The molarity of the copper solution in the cuvette was determined by spectrophotometric analysis to be 2.90×10−2 M Cu. Calculate the mass of copper in the Jefferson nickel used to make the first solution.
Answers
The mass of copper in the Jefferson nickel used to make the first solution is approximately 1.84 grams after using the molarity of the copper solution in the cuvette, the volume of the aliquot, and the dilution factor.
Given:
Molarity of the copper solution = 2.90×10^−2 M Cu
Volume of the aliquot = 5.00 mL
Dilution factor = Volume of second solution / Volume of aliquot = 100.00 mL / 5.00 mL = 20
Step 1: Calculate the moles of copper in the aliquot.
Moles of copper = Molarity × Volume = (2.90×10^−2 M) × (5.00 mL / 1000 mL/ L) = 1.45×10^−3 moles
Step 2: Calculate the moles of copper in the first solution.
Moles of copper in the first solution = Moles of copper in the aliquot × Dilution factor = (1.45×10^−3 moles) × 20 = 2.90×10^−2 moles
Step 3: Calculate the mass of copper in the Jefferson nickel.
Mass of copper = Moles of copper × Molar mass of copper
The molar mass of copper is approximately 63.55 g/mol.
Mass of copper = (2.90×10^−2 moles) × (63.55 g/mol) ≈ 1.84 g
In this calculation, we first determine the moles of copper in the aliquot by multiplying the molarity of the copper solution by the volume of the aliquot in liters. Then, we use the dilution factor to calculate the moles of copper in the first solution by multiplying the moles in the aliquot by the dilution factor. Finally, we find the mass of copper by multiplying the moles of copper by the molar mass of copper.
It's important to note that the molarity of the copper solution is determined through spectrophotometric analysis, which measures the absorbance of light by the copper solution and relates it to concentration. The dilution factor is used to account for the dilution of the original solution when preparing the second solution. By following these calculations, we can estimate the mass of copper in the Jefferson nickel used to make the first solution.
To know more about dilution factor, please click on:
https://brainly.com/question/30887569
#SPJ11
Which physical property is used to be Indentify the ability to dissolve
Answers
Answer:
solubility
Explanation:
solubility is a measure of how much a substance will dissolve in a solvent.
If a wave is traveling at a steady speed and its frequency is
doubled, what happens to the wavelength of that wave?
Answers
Answer:
If the frequency is doubled, the wavelength is only half as long.
Explanation:
Wave speed equals frequency*wavelength. So doubling the frequency must halve the wavelength in order for wave speed to remain the same.
Physical properties are used to classify and compare substances. Physical
properties can include density, thermal conductivity, electrical conductivity,
solubility, magnetism, melting point, and boiling point. Which statement is
correct about all of these physical properties?
They involve a change in the state of matter.
They involve the production of new substances.
They can be measured only after a change in state.
They are independent of the amount of the sample present.
Answers
Answer:
Physical properties involve a change in the state of matter.
Explanation:
A chemical change would change the chemical composition.
A physical change would change the state of matter, for example solid to liquid to gas are changes in state. So, this means when water changes states of matter it is a physical change.
What is sampling procedure in Research example?.
Answers
Sampling is the process of choosing the group from from which you'll actually collect data for your study.
What does chemical research entail?Chemistry undergraduate research is independent experimentation carried out under the direction and supervision of something like a mentor or adviser. In an ongoing study project, students look at topics that interest them and their adviser. The chemical sciences cover a wide range of subject areas.
What kinds of research are there in chemistry?Modern chemistry research may be divided into two categories: pure and applied. Pure chemistry researchers work primarily to improve human knowledge of chemistry. Greater comprehension of the ideas behind how matter changes during chemical reactions is what pure chemistry is all about.
To know more about Research visit:
https://brainly.com/question/3628862
#SPJ4
calculate the concentration of h3o ions present in a solution of hcl that has a measured ph of 1.510 .
Answers
The concentration of H3O+ ions in the solution of HCl is 3.72 x 10^(-2) M.
The pH of a solution is defined as the negative logarithm (base 10) of the hydronium ion concentration, or [H3O+].
Mathematically, we can express this relationship as:
pH = -log[H3O+]
Therefore, we can rearrange this equation to solve for [H3O+]:
[H3O+] = 10^(-pH)
Substituting the given pH of 1.510 into this equation, we get:
[H3O+] = 10^(-1.510)
[H3O+] = 3.72 x 10^(-2) M
Therefore, the concentration of H3O+ ions in the solution of HCl is 3.72 x 10^(-2) M.
Learn more about concentration, here:
https://brainly.com/question/3045247
#SPJ11
Consider the reaction 2CuCl2 + 4KI → 2CuI + 4KCl + I2. If 4 moles of CuCl2 react with 4 moles of KI, what is the limiting reactant?
Answers
Answer:
The limiting Reactant would be KI
Explanation:
Fusion is safer than fussion because....
A. a fusion reaction will stop on its own if something goes wrong
B. a fusion recrion creates waste material that is easier to store than fission
Answers
Answer: B, a fusion recrion creates waste material that is easier to store than fission.
Fusion produces far less harmful waste than fission does. The reliance of something independent like whether a reaction will stop on its own when something goes wrong is never indefinite (technological failures are unpredictable and destructive a lot of the time when it comes down to nuclear power).
What is the empirical formula for a compound that has 1. 5 moles of copper and 0. 5 moles of phosphorus
Answers
1.5 moles of copper and 0.5 moles of
phosphorus is CuP.
A team of botanists conducted an experiment
investigating the effect of pH on plant growth.
The height of the plant was measured three weeks
after planting.
1
?
3.
Based on the data they collected, what is the
optimal pH for growing basil? Explain your
answer.
Based on the data they collected, which
plant fares better than the others in low pH
environments? Explain your answer.
At which pH is there the greatest difference
between the heights of parsley and basil?
What is the height difference at that pH?

Answers
The outcomes to the scan had been now not all similar. The pots with the pH of 5.0 had no growth whatsoever. The pots with the pH of 6.0 had little growth, each with only four blades of grass. The pots with a pH of 7.0 grew well, one pot with extra blades of grass than the other, an average of 11 blades of grass
What are the elements that affect the pH of a plant environment?Natural soil pH depends on the rock from which the soil was once fashioned (parent material) and the weathering procedures that acted on it—for instance climate, vegetation, topography and time. These approaches have a tendency to purpose a decreasing of pH (increase in acidity) over time.
There is disruption of nutrient absorption by way of the plants if it's pH increases, and hence, soil fertility is reduced, alkaline soil's pH does not lead to make bigger in nutrient absorption, soil illness does not happen.
Learn more about effect of pH on plant growth here:
https://brainly.com/question/31459436#SPJ12. What are the units for the mass of a solid? mass of a liquid?
Answers
Answer:
I'm not sure myself but I'd say mass since it's a solid
When 13.4 g of an unknown, non-volatile, non-electrolyte, X was dissolved in 100. g of benzene, the vapor pressure of the solvent decreased from 100 torr to 90.9 torr at 299 K. Calculate the molar mass of the solute, X.
Answers
The molar mass of the solute, X is 35.37 g/mol. We used the formula to calculate the molar mass of the solute, X. We also used Raoult's Law to find out the mole fraction of the solute, XB. We then substituted the given values in the formula to find out the molar mass of the solute, X.
When 13.4 g of an unknown, non-volatile, non-electrolyte, X was dissolved in 100. g of benzene, the vapor pressure of the solvent decreased from 100 torr to 90.9 torr at 299 K. Calculate the molar mass of the solute, X, Molar mass is defined as the mass of one mole of a substance. The formula used to calculate molar mass is as follows: Molar mass (M) = Mass (m) ÷ Number of moles (n)For non-volatile, non-electrolyte solutions, the formula used to calculate the molar mass of the solute is as follows: P = P°A × XB × Kb × nA/ V bwhere, P is the change in the vapor pressure of the solvent P°A is the vapor pressure of the pure solvent XB is the mole fraction of the solute Kb is the molal boiling point elevation constant nA is the number of moles of the solute Vb is the volume of the solvent in kg The given data is: Molar mass of the solute, X = Mass of the solute, X = 13.4 g Mass of the solvent, benzene = 100 g Change in vapor pressure, ΔP = 100 - 90.9 = 9.1 torr Vapor pressure of the pure solvent, P°A = 100 torr Temperature, T = 299 K Molal boiling point elevation constant, Kb = 2.53 °C/m Volume of the solvent in kg, Vb = 100/1000 = 0.1 kg We can use Raoult's Law to find out the mole fraction of the solute, XB. It states that the vapor pressure of the solvent in a solution is directly proportional to the mole fraction of the solvent present in the solution. Mathematically, it can be represented as: P = PA° × XA where, P is the vapor pressure of the solution PA° is the vapor pressure of the pure solvent XA is the mole fraction of the solvent in the solution The mole fraction of the solute, XB is given by: XB = 1 - XA The mole fraction of the solvent, XA can be calculated as follows :XA = PA/P°A = (100 - 9.1)/100 = 0.909XB = 1 - XA = 1 - 0.909 = 0.091Now, we can substitute the given values in the formula to find out the molar mass of the solute, X.ΔP = P°A × XB × Kb × nA/Vb9.1 = 100 × 0.091 × 2.53 × nA/0.1nA = 0.3788 moles Molar mass (M) = Mass (m) ÷ Number of moles (n)M = 13.4 ÷ 0.3788M = 35.37 g/mol Therefore, the molar mass of the solute, X is 35.37 g/mol.
In this question, we are given the mass of an unknown, non-volatile, non-electrolyte solute, X and the vapor pressure of the solvent, benzene at two different temperatures. We are required to calculate the molar mass of the solute, X. The molar mass of a substance is defined as the mass of one mole of the substance. It is usually expressed in g/mol. The formula used to calculate molar mass is as follows: Molar mass (M) = Mass (m) ÷ Number of moles (n)We are given the mass of the solute, X as 13.4 g. Therefore, we need to find the number of moles of the solute, X to calculate its molar mass. We can use the formula to calculate the molar mass of the solute if we know its boiling point elevation constant, Kb, molality, temperature, volume of the solvent in kg, and change in vapor pressure. The solute is non-volatile, non-electrolyte. Therefore, we can use Raoult's Law to find out the mole fraction of the solute, XB. It states that the vapor pressure of the solvent in a solution is directly proportional to the mole fraction of the solvent present in the solution. Mathematically, it can be represented as: P = PA° × XA where, P is the vapor pressure of the solution PA° is the vapor pressure of the pure solvent XA is the mole fraction of the solvent in the solution We can find out the mole fraction of the solvent, XA as follows: XA = PA/P°A = (100 - 9.1)/100 = 0.909We can find out the mole fraction of the solute, XB as follows: XB = 1 - XA = 1 - 0.909 = 0.091 Now, we can substitute the given values in the formula to find out the molar mass of the solute, X.ΔP = P°A × XB × Kb × nA/Vb9.1 = 100 × 0.091 × 2.53 × n A/0.1nA = 0.3788 moles Molar mass (M) = Mass (m) ÷ Number of moles (n)M = 13.4 ÷ 0.3788M = 35.37 g/mol Therefore, the molar mass of the solute, X is 35.37 g/mol.
The molar mass of the solute, X is 35.37 g/mol. We used the formula to calculate the molar mass of the solute, X. We also used Raoult's Law to find out the mole fraction of the solute, XB. We then substituted the given values in the formula to find out the molar mass of the solute, X.
To know more about Raoult's Law visit:
brainly.com/question/2253962
#SPJ11
the gas in a 225.0 ml piston experiences a change in pressure from 1.00 atm to 2.90 atm. what is the new volume (in ml) assuming the moles of gas and temperature are held constant?
Answers
When a gas is subjected to changes in pressure, volume, or temperature, its properties change. However, when moles of gas and temperature are held constant, the only property that changes is the volume of the gas. In this case, the gas in a 225.0 ml piston experiences a change in pressure from 1.00 atm to 2.90 atm, which means the volume of the gas must have decreased.
Boyle's Law states that for a given amount of gas at constant temperature, the product of the pressure and volume is constant. Mathematically, this is represented as P1V1 = P2V2, where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume.
Given:
Initial pressure (P1) = 1.00 atm
Initial volume (V1) = 225.0 mL
Final pressure (P2) = 2.90 atm
We need to find the new volume (V2).
Using Boyle's Law, P1V1 = P2V2:
(1.00 atm) * (225.0 mL) = (2.90 atm) * V2
Now, solve for V2:
V2 = (1.00 atm * 225.0 mL) / (2.90 atm)
V2 ≈ 77.6 mL
So, when the pressure changes from 1.00 atm to 2.90 atm, the new volume of the gas in the piston is approximately 77.6 mL, assuming the moles of gas and temperature are held constant.
For more information on Boyle's Law see:
https://brainly.com/question/30367067
#SPJ11
/Why does an atom want all of it’s orbitals filled
Why does it want to be in a stable state
Answers
Smartphone batteries contain a casing that surrounds it that is made up out of aluminium. What would best describe the properties of aluminium that enables it to be used for such purposes?
Answers
Aluminium is chosen for smartphone battery casings due to its light weight, durability, and corrosion resistance.
Aluminium is a lightweight metal, making it ideal for smartphone battery casings as it helps keep the overall weight of the device down. Additionally, aluminium is known for its durability, meaning it can withstand everyday wear and tear without easily getting damaged. This is important for protecting the battery and ensuring its longevity.
Furthermore, aluminium is highly resistant to corrosion, which means it is less likely to rust or degrade over time, even when exposed to moisture or other environmental factors. This corrosion resistance helps maintain the integrity and functionality of the battery casing, ensuring the safety and reliability of the smartphone. Overall, the combination of its light weight, durability, and corrosion resistance makes aluminium a suitable material for smartphone battery casings.
Learn more about corrosion here:
https://brainly.com/question/33356820
#SPJ11
As a result of this process, the proportions of oxygen and carbon dioxide in
air breathed in and air breathed out change.
Which one of the statements is true? Tick the correct box. [1]
- Air breathed out has less carbon dioxide and more oxygen than air breathed in.
- Air breathed out has less carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and less oxygen than air breathed in.
- Air breathed out has more carbon dioxide and more oxygen than air breathed in.
Answers
Answer:
the third one
Explanation:
When you breathe in, you inhale oxygen and exhale carbon dioxide
need help i will give you thanks:)

Answers
Answer:
Precipitation
Explanation:
Precipitation is when water falls from the clouds back to earth as rain, snow, or hail. The water returns to the rivers to continue the water cycle. So, the answer is D, precipitation.
I hope this helps :)
Answer:
Vapor Information
Explanation:
Atmospheric rivers are relatively long, narrow regions in the atmosphere like rivers in the sky that transport most of the water vapor outside of the tropics. Not all atmospheric rivers cause damage, and most are weak systems that often provide beneficial rain or snow that is crucial to the water supply.
(You're welcome and have a nice day/night Ma'am/Sir.)
Nickel has a cubic unit cell. The edge of the unit cell is 3.524
x 10^(-8)cm. Determine the atomic radius of Nickel.
Answers
The approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
In a cubic unit cell, the body diagonal length (diagonal that passes through the center of the unit cell) is equal to four times the atomic radius (4r). We can use this relationship to find the atomic radius of nickel.
Given: Edge length of the unit cell (a) = 3.524 × 10^(-8) cm
The body diagonal length is given by:
Diagonal length (d) = a√3
Substituting the given values:
d = (3.524 × 10^(-8) cm) × √3
Now, we can calculate the atomic radius (r) by dividing the diagonal length by 4:
r = d / 4
Performing the calculations:
r = [(3.524 × 10^(-8) cm) × √3] / 4
r ≈ (3.524 × 10^(-8) cm) × (1.732 / 4)
r ≈ 1.532 × 10^(-8) cm
Therefore, the approximate atomic radius of nickel is 1.532 × 10^(-8) cm.
Know more about atomic radius here
https://brainly.com/question/18095927#
#SPJ11
Outdoor decay of objects is a chemical reaction. Some ancient objects have been preserved extremely well in Arctic conditions. As glaciers have melted, archaeologists have discovered 4,000-year-old Viking clothing. Clarissa left her wool sweater outside at her family's summer cottage in Florida. When she returned the following summer, the sweater was in shreds, decayed by reactions that break down wool. Based on the data in the table, what can you conclude about the rate at which clothing decays in cold temperatures compared with the rate at which clothing decays in warm temperatures?
A. More information is needed to compare rates of decay
B. Reactions occur at the same speed in all temperatures
C. Reactions occur more quickly in the cold
D. Reactions occur more slowly in the cold.

Answers
Answer: D. Reactions occur more slowly in the cold.
Explanation:

Long chain and very long chain FA require ____________ to enter the mitochondrial matrix for beta-oxidation
Answers
Long chain and very long chain fatty acids (FA) require carnitine shuttle system to enter the mitochondrial matrix for beta-oxidation.
This system consists of three primary components: carnitine palmitoyltransferase I (CPT I), carnitine-acylcarnitine translocase (CACT), and carnitine palmitoyltransferase II (CPT II). CPT I, located on the outer mitochondrial membrane, converts the long-chain FA into their respective acylcarnitines by attaching a carnitine molecule to them. These acylcarnitines can then be transported across the inner mitochondrial membrane by CACT, which is a transport protein. Once inside the matrix, CPT II, which is bound to the inner mitochondrial membrane, detaches the carnitine group and reattaches the original CoA group, generating a long-chain acyl-CoA that is ready for beta-oxidation.
Beta-oxidation is a process that breaks down fatty acids into smaller units called acetyl-CoA, which can then enter the citric acid cycle (also known as the Krebs cycle or TCA cycle) to generate ATP, the energy currency of cells. This process is vital for energy production, especially during times of fasting or prolonged exercise when glucose stores are depleted. Overall, the carnitine shuttle system is essential for the efficient transport and utilization of long chain and very long chain fatty acids for energy production through beta-oxidation.
To learn more about beta-oxidation here:
https://brainly.com/question/29458295
#SPJ11
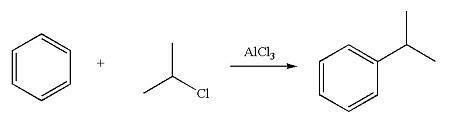
What product is formed when benzene is treated with the following organic halide in the presence of alcl3? click the draw structure button to launch the drawing utility.
Answers
Cumene is the end result of the reaction between benzene and 2-chloropropane when aluminum chloride is present.
Alkylation serves as an example of replacing the "alkyl group on the aromatic ring." In the alkylation reaction, the term "alkyl halides" is important. The "Lewis acid" suggested an alkylation catalyst.
Cumene is produced when the benzene molecule interacts with 2-chloropropane in the presence of aluminum chloride. By losing chlorine, the 2-chloropropane combines with aluminum chloride to produce a carbocation. Cumene is produced by further attacking benzene and protonating it.
Below is an attachment showing the cumene generation reaction.
Cumene is the end result of the reaction between benzene and 2-chloropropane when aluminum chloride is present.
Learn more about cumene here-
https://brainly.com/question/13136313
#SPJ4
Why do some bodies in the Solar System maintain countless well-preserved indications of impacts on their surface
even after many millions of years? Give some examples and explain in detail how at least 2 factors contribute to this
Answers
Answer:
There are several factors that contribute to the preservation of impact craters on the surface of bodies in the Solar System.
One factor is the lack of erosion or weathering on these bodies. Many of the bodies in the Solar System, such as the Moon and many of the outer planets' moons, do not have atmospheres or hydrospheric systems (like oceans, lakes, or rivers) that can erode or weather the surface over time. This means that the surface of these bodies is not subjected to the same types of processes that can wear away or smooth out surface features on Earth. As a result, impact craters and other surface features can remain well-preserved for millions of years.
Another factor that can contribute to the preservation of impact craters is the presence of a surface layer of material that is resistant to erosion. For example, on the Moon, the surface is covered by a layer of fine-grained dust called regolith, which is made up of small rocks and particles that have been ground up by impact and other processes. This regolith layer is relatively soft and easily disturbed, but it also acts as a protective layer that can preserve the underlying surface features. Similarly, on some of the outer planets' moons, there may be a layer of ice or other materials that can act as a protective layer and preserve impact craters and other surface features.
Examples of bodies in the Solar System that have well-preserved impact craters include the Moon, Mars, and many of the outer planets' moons, such as Europa and Ganymede. The lack of erosion and the presence of protective surface layers on these bodies have allowed them to maintain countless well-preserved indications of impacts on their surfaces even after many millions of years.
Explanation:
To solve this, we must know each and every concept behind solar system. Therefore, impact craters enable scientists to investigate the geological history of a planet.
What is solar system?The Solar System is composed of the Sun and the objects that orbit it. It was created by the gravitational collapse of a massive interstellar molecular cloud 4.6 billion years ago. The Sun contains the vast bulk of the system's mass (99.86%)
Several processes contribute to the preservation of impact craters on the surfaces of Solar System worlds. The absence of erosion or weathering on these bodies is one cause. Another aspect that can aid in the preservation of impact craters is the existence of an erosion-resistant surface layer of material.
Therefore, impact craters enable scientists to investigate the geological history of a planet.
To know more about solar system, here:
https://brainly.com/question/18365761
#SPJ2
Manny designs an experiment to learn about erosion. First, he sets up three long trays with equal-sized piles of sand at one of each tray. Then he sets up Tray A so that it lays flat on a table. He elevates the end of Tray B that holds the sand by 5 cm. Next, he elevates the end of Tray C that holds the sand in each tray. Finally, he observes and measures how far sand is carried by the way water poured into each tray.
Answers
Answer:
Height of tray
Explanation:
I don’t know what the correct answer is? Can anyone explain?

Answers
Short Answer:
C)
Long Answer:
A- When a solid is heated, it melts and becomes a liquid. (Another older term is Fusion.) An example of this is ice to water when you leave it outside the freezer.
B- When a liquid becomes a gas, it undergoes a process called boiling. Example: When you boil water, it becomes steam.
C- It is called deposition. Example: How snow forms in clouds.
in the section on nuclear stability, two nuclear processes are described - nuclear fusion and nuclear fission. one takes place in our sun as four hydrogen atoms combine to create a single atom of helium. the other is an example of how a controlled reaction creates energy in a nuclear power plant. which is which? in the section on nuclear stability, two nuclear processes are described - nuclear fusion and nuclear fission. one takes place in our sun as four hydrogen atoms combine to create a single atom of helium. the other is an example of how a controlled reaction creates energy in a nuclear power plant. which is which?
Answers
Nuclear fusion is the process that takes place in our sun as four hydrogen atoms combine to create a single atom of helium. This is the process by which stars generate their energy, and it is an extremely powerful reaction that releases vast amounts of energy.
Nuclear fusion is a process in which two atomic nuclei combine to form a heavier nucleus, releasing a large amount of energy in the process. This process is what powers the sun and other stars, where the intense pressure and temperature at the core allow for the fusion of hydrogen atoms into helium.
Scientists have been trying to replicate this process on Earth in order to harness the enormous energy potential of fusion. However, the challenges are significant as it requires high temperatures and pressures to overcome the electrostatic repulsion between positively charged nuclei. If we can successfully achieve nuclear fusion, it could provide a virtually limitless source of clean energy without producing harmful greenhouse gas emissions or radioactive waste.
To know more about Nuclear fusion refer to-
brainly.com/question/14019172
#SPJ4
.Which electrolyte deficiency triggers the secretion of renin?
1-Sodium
2-Calcium
3-Chloride
4-Potassium
Answers
The electrolyte deficiency of Sodium triggers the secretion of renin. The answer is 1.
The deficiency of sodium triggers the secretion of renin. Renin is an enzyme produced and released by special cells in the kidneys called juxtaglomerular cells. It plays a key role in regulating blood pressure and fluid balance in the body. When the sodium levels in the body are low, it signals the juxtaglomerular cells to release renin into the bloodstream.
Renin then initiates a series of biochemical reactions that ultimately lead to the production of angiotensin II, a potent vasoconstrictor. Angiotensin II causes blood vessels to constrict, which helps to increase blood pressure.
Therefore, the answer is 1.
To know more about electrolyte deficiency, refer here:
https://brainly.com/question/32080991
#SPJ11
A deficiency in Sodium triggers the secretion of renin, a hormone released by the kidneys that acts to increase sodium reabsorption to maintain blood volume and blood pressure.
Explanation:The electrolyte deficiency which triggers the secretion of renin is Sodium. The kidneys regulate electrolyte levels and when there is a deficiency in sodium, this signals a need for increased blood volume. The decrease in sodium level stimulates the release of renin, a hormone released by the kidneys, which then activates the renin-angiotensin-aldosterone system (RAAS). This system acts to increase sodium reabsorption, helping to elevate blood volume and blood pressure.
Learn more about Renin secretion here:https://brainly.com/question/32325669
#SPJ11
Question 2 of 15
Which statement is true of both nuclear fusion and nuclear fission?
A. The nucleons in the resulting nuclei differ from the nucleons in the
original nuclei.
B. The products are nuclei of elements that are different from the
original elements.
C. All the nuclei involved in the nuclear reaction represent the same
element.
D. The nucleons in the original nuclei are destroyed by the process.
Answers
Answer:
B. The products are nuclei of elements that are different from the original elements.