50.0 mL of an HCl solution with a pH of 3.5 neutralizes 200.0 mL of a Ca(OH)2 solution. What is the molarity of the Ca(OH)2 solution
Answers
50.0 mL of an HCl solution with a pH of 3.5 neutralizes 200.0 mL of a Ca(OH)₂ solution. Molarity of this Ca(OH)₂ solution is 7.90 x 10⁻⁵ M.
As per the given information;
the neutralization of HCl with Ca(OH)₂ can be written as;
HCl + Ca(OH)₂ → CaCl₂ + 2H₂O
Since HCl is monoprotic acid, 1 mole of HCl reacts with 1 mole of Ca(OH)₂.
Thus, the molarity of HCl can be calculated as;
Molarity = (moles of HCl) / (volume of HCl in Liters)
Since, pH of HCl is given as 3.5, it means; [H+] = 10^(-pH) = 10⁻³·⁵ = 3.16 x 10⁻⁴
Molarity of HCl = [HCl] = [Cl-] = 3.16 x 10⁻⁴ M (due to complete ionization of HCl in water)
Thus, moles of HCl in 50 mL of HCl solution = (3.16 x 10⁻⁴ mol/L) × (50 mL / 1000 mL/L) = 1.58 x 10⁻⁵ moles of HCl
Now, as per the reaction; 1 mole of HCl reacts with 1 mole of Ca(OH)₂,So, 1.58 x 10⁻⁵ moles of HCl reacts with 1.58 x 10⁻⁵ moles of Ca(OH)₂.
Now, the volume of Ca(OH)₂ solution is given as 200.0 mL.
Thus, the concentration of Ca(OH)₂ solution can be calculated as;Molarity = (moles of Ca(OH)₂) / (volume of Ca(OH)₂ solution in Litres)
Molarity = (1.58 x 10⁻⁵ moles of Ca(OH)₂) / (200.0 mL / 1000 mL/L) = 7.90 x 10⁻⁵ M
Thus, the molarity of Ca(OH)₂ solution is 7.90 x 10⁻⁵ M.
For more questions on Molarity of Ca(OH)₂ :https://brainly.com/question/3276733
#SPJ11
Related Questions
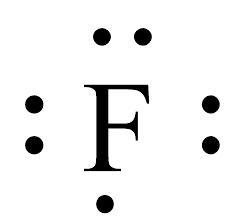
Draw the correct Lewis dot structure from the given shorthand notation below: PLS HELP

Answers
The Lewis structure of the element have been shown in the image attached.
Lewis dot structure of an element:The valence electrons of an atom or molecule are depicted in a simplified manner by the Lewis structure, commonly referred to as the Lewis dot structure or electron dot structure. Gilbert N. Lewis, an American scientist, created it.
The valence electrons of an atom are shown in a Lewis structure as dots surrounding the element's symbol. These dots' placement reveals details about the connectivity and atom-atom bonding in a molecule.
Learn more about Lewis structure:https://brainly.com/question/29756546
#SPJ1
Calculate the ph of a 0.377 m solution of ethylenediamine ( h2nch2ch2nh2 ). the pa values for the acidic form of ethylenediamine ( h 3nch2ch2nh 3 ) are 6.848 ( pa1 ) and 9.928 ( pa2 ).
Answers
The final answer is 11.71
The concentration of a given solution (H2NCH2CH2NH2) is 0.314 M
pa1 = 6.848
a1 = 1.42 X \(10^{-7}\)
Pa2 = 9.928
a2 = 1.18 X \(10^{-10}\)
The equation is given as;
NH2CH2CH2NH2 + H2O ---> NH2CH2CH2NH3+ + OH-
Initial 0.314 0 0
Change -x +x +x
Equilibrium 0.314 -x x x
b1 = Kw / Ka2 = 10^-14 / 1.18 X 10^-10 = 8.47 X 10^-5 = [NH2CH2CH2NH3+][OH-] / [NH2CH2CH2NH2]
8.47 X 10^-5 = x^2 / 0.314 -x
8.47 X 10^-5 X 0.314 - 8.47 X 10^-5x = x^2
x^2 - 2.66 X 10^-5 + 8.47 X 10^-5x = 0
on solving
x = 0.0051 M = [NH2CH2CH2NH3+] = [OH-]
[NH2CH2CH2NH2] = 0.314 - 0.0051 = 0.3089 M
For the second dissociation,
NH2CH2CH2NH3+ + H2O ---> +NH3CH2CH2NH3+ + OH-
Initial 0.0051 0 0.0051
Change -x +x +x
Equilibrium 0.0051 -x x 0.0051 +x
b2 = Kw / Ka1 = 10^-14 / 1.42 X 10^-7 = 7.04 X 10^-8 = [+NH3CH2CH2NH3+] [OH-] / [+NH3CH2CH2NH2]
7.04 X 10^-8 = x (0.0051+x) / (0.0051-x)
We may ignore x in the denominator as b2 is very low
7.04 X 10^-8 = x (0.0051+x) / (0.0051)
3.59 X 10^-10 = 0.0051x + x^2
x = 7.039 X 10^-8 M
[OH-] = 0.0051 + x = 0.0051 approx
[+NH3CH2CH2NH3+] = x = 7.039 X 10^-8 M
NH2CH2CH2NH3+ = 0.0051 +x = 0.0051 M approx
pOH = --log [OH-] = -log 0.0051 = 2.29
pH = 14 - pOH = 11.71
Learn more about ph: https://brainly.com/question/12609985
#SPJ4
How many moles do you have if you have 144 L of a gas at SATP?

Answers
Answer
moles = 5.81 mol
Explanation
Given:
Volume = 144 L
AT SATP
1 mole = 24.4651 L
Solution:
1 mole = 24.4651 L
x mole = 144 L
x = 144/24.4651
x = 5.8 mol
what type of energy do ethanol, propane, and butane all have?
Answers
Ethanol, propane, and butane are all examples of fuels that contain chemical energy. This chemical energy is stored within the molecules of the fuel and can be released during a chemical reaction, such as combustion, to produce heat and/or light energy.
When ethanol, propane, or butane is burned, the chemical bonds between the atoms within the molecule are broken, releasing energy in the form of heat and light. This energy can then be harnessed for various purposes, such as heating buildings or powering vehicles. Overall, the energy content of a fuel depends on the specific molecules it contains and their chemical structures.
To know more about Ethanol click this link -
brainly.com/question/25002448
#SPJ11
Question 11
Which formula represents a hydrocarbon?
C₂H6
C₂H5OH
C₂H5Cl
C₂H6O
Answers
Answer:
C₂H6
Explanation:
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). Option A
A hydrocarbon is a compound that consists of only carbon and hydrogen atoms. It is important to identify the formula that represents a hydrocarbon among the given options:
A) C₂H6: This formula represents ethane, which is a hydrocarbon. Ethane consists of two carbon atoms bonded together with single bonds and six hydrogen atoms.
B) C₂H5OH: This formula represents ethanol, which is not a hydrocarbon. Ethanol contains a hydroxyl group (-OH), indicating the presence of oxygen in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
C) C₂H5Cl: This formula represents ethyl chloride, which is not a hydrocarbon. Ethyl chloride contains a chlorine atom (Cl) in addition to carbon and hydrogen atoms. It is a haloalkane, not a hydrocarbon.
D) C₂H6O: This formula represents ethanol, which, as mentioned before, is not a hydrocarbon. Ethanol contains an oxygen atom (O) in addition to carbon and hydrogen atoms. It is an alcohol, not a hydrocarbon.
Among the given options, the formula A) C₂H6 represents a hydrocarbon (specifically, ethane). It consists only of carbon and hydrogen atoms, making it a suitable representation of a hydrocarbon.
In summary, the formula C₂H6 (option A) represents a hydrocarbon, while the other options contain additional elements (oxygen or chlorine) that make them non-hydrocarbon compounds. Option A
For more such questions on hydrocarbon visit:
https://brainly.com/question/21281906
#SPJ8
If you have 547.3 grams of Ni2O, how many molecules would be present?
Answers
Answer: 8.830418848725065
Explanation:
8.830418848725065
A building is found to contain a radioactive gas. Thirty hours later, 60% of the initat amount of gas is still present. (Note: Use the concepts or haif-ife or doubling time.) (a) Write a model for the percentage of the initial atnount of the radioactive gas present after t hours. (Round all numerical values to three decimal pisces.) r(t)= (b) Calculate the balfelife of the radioactive gas. (Round your anwwer to three decimal places.) hours
Answers
A building is found to contain a radioactive gas. Thirty hours later, 60% of the initial amount of gas is still present.So, we have to calculate the half-life of the radioactive gas.
(a) Write a model for the percentage of the initial amount of the radioactive gas present after t hours. (Round all numerical values to three decimal places.)
Formula used to find the amount of radioactive gas present after t hours:
(t) = r₀ (1/2)^(t/T)Here,r₀ = initial amount of the radioactive gasr(t) = amount of radioactive gas present after t hoursT = half-life of the radioactive gasGiven, 60% of the initial amount of gas is still present after thirty hours.So, the remaining amount of radioactive gas is 40%.That is, r(t) = 40% = 0.40r₀and t = 30So, the formula becomes:
0.40 = r₀ (1/2)^(30/T)Multiply both sides by 2^30,0.40(2^30) = r₀(2^30) (1/2)^(30/T)0.40(2^30) = r₀(1/2)^(30/T-30)0.40(2^30) = r₀(1/2)^(t/T - 1)Therefore,r(t) = r₀ (1/2)^(t/T) = 0.40 (1/2)^(t/T - 1)Hence, r(t) = 0.4 x (0.5)^(t/T - 1).(b) Calculate the half-life of the radioactive gas. (Round your answer to three decimal places.)From the formula,r(t) = r₀ (1/2)^(t/T)When t = T, r(t) = 1/2 of r₀r(T) = r₀/2Divide both sides by r₀r(T)/r₀ = 1/2We have r(T)/r₀ = 0.5, and the formula becomes 0.5 = (1/2)^(T/T)0.5 = (1/2)^1T = 1Therefore, the half-life of the radioactive gas is one hour.Answer Half-life of the radioactive gas = 1 hour.
About RadioactiveRadioactive is the ability of an unstable atomic nucleus to become stable through emission of radiation. This capability involves the process of splitting unstable atomic nuclei resulting in energy loss by emitting radiation, such as alpha particles, beta particles with neutrinos and gamma rays. What is radioactive impact? It is necessary to know, radioactive substances from nuclear radiation are compounds that are harmful to humans and other living things. The effects of nuclear radiation not only damage human DNA, but can also cause cancer. The effects of nuclear radiation can damage the atoms in the body and damage DNA.
Learn More About Radioactive at https://brainly.com/question/1236735
#SPJ11
Sort the following molecules based on whether or not they are biologically occurring ketone bodies. Drag each item to the appropriate bin.View Available Hint(s) Reset Help B-hydroxybutyrate ethyl methyl ketone acetone acetoacetate 2-butanone 2.pontanone Biological ketone bodies Nonbiological IcetonesPrevious question
Answers
Biological ketone bodies are Acetone, B-hydroxy butyrate , acetoacetate and non biological ketone bodies are Ethyl methyl ketone, 2-butanone and 2-pentanone.
Biological ketone bodies: Water soluble ketones produced by liver during the process of gluconeogenesis. Ketone bodies or simply ketones are substances produced by the liver during gluconeogenesis. This is a process that creates glucose in times of fasting and starvation. The biological ketone bodies are Acetone, B-hydroxy butyrate , acetoacetate.
Non-biological ketone: The ketones which are not generated by liver during the gluconeogenesis. Ketone bodies are water soluble molecules that contain the ketone groups produced from fatty acids by the liver. Ketone bodies are readily transported into tissues outside the liver where they are converted into acetyl-CoA which then enters the citric acid cycle and is oxidized for energy. Examples are Ethyl methyl ketone, 2-butanone and 2-pentanone.
To learn more about Gluconeogenesis please visit:
https://brainly.com/question/1425339
#SPJ4
Reproductions is the process by which organisms CANNOT make one or more organisms
True
False
Answers
devise a synthesis of the following biologically active compound from benzene. draw the intermediates and select the correct reagent for each step of the reaction sequence.
Answers
We can synthesize acetaminophen from benzene. Please note that the specific reagents and reaction conditions may vary depending on the desired biologically active compound.
To synthesize a biologically active compound from benzene, we need to plan a reaction sequence using appropriate reagents. Let's consider an example where we want to synthesize acetaminophen from benzene.
Step 1: Convert benzene to nitrobenzene
- Reagent: Concentrated nitric acid (HNO_3) and concentrated sulfuric acid (H_2SO_4)
- Reaction: Nitration
- Mechanism: Benzene undergoes electrophilic aromatic substitution with nitric acid, resulting in the formation of nitrobenzene.
Step 2: Reduce nitrobenzene to aniline
- Reagent: Hydrogen gas (H_2) and a reducing agent like iron (Fe) or tin (Sn)
- Reaction: Reduction
- Mechanism: Nitrobenzene is reduced to aniline by catalytic hydrogenation, using a metal catalyst such as iron or tin.
Step 3: Acetylate aniline to form acetanilide
- Reagent: Acetic anhydride (CH_3CO)_2O
- Reaction: Acetylation
- Mechanism: Aniline reacts with acetic anhydride, resulting in the formation of acetanilide.
Step 4: Convert acetanilide to acetaminophen
- Reagent: Sodium hydroxide (NaOH) and acetic anhydride (CH_3CO)_2O
- Reaction: Hydrolysis and acetylation
- Mechanism: Acetanilide undergoes hydrolysis with sodium hydroxide to form aniline. The resulting aniline is then acetylated using acetic anhydride, resulting in the formation of acetaminophen.
By following this reaction sequence, we can synthesize acetaminophen from benzene. Please note that the specific reagents and reaction conditions may vary depending on the desired biologically active compound.
Learn more about nitric acid from the following link:
https://brainly.com/question/2269846
#SPJ11
how many moles of MgO can be made from 10 moles of fe2O3?
Answers
Answer:
Fe 2 O3 .
Explanation:
you are given 0.856 g of an unknown diprotic acid, h2a. it reacts with naoh according to this balanced equation: h2a(aq) 2 naoh(aq) na2a(aq) 2 h2o(l) if a volume of 30.8 ml of 0.515 m naoh is required to react with all of the acid, what is the molar mass of the acid?
Answers
molar mass of acid = 0.0345 moles acid x molar mass of acid = 306.325 g/mol.
What is molar mass ?Molar mass is a physical property of a substance that is defined as the mass of one mole of the substance, and is expressed in grams per mole (g/mol). It is also known as molecular weight and is equal to the sum of the atomic masses of all the atoms in a molecule. The molar mass is used in many calculations when dealing with solutions and thermodynamics.
The balanced equation tells us that for every mole of acid, two moles of NaOH are needed. We can therefore use the information we have to calculate the number of moles of NaOH in the given volume:
30.8 mL x 0.515 M NaOH = 15.742 moles NaOH
We can also calculate the number of moles of acid:
0.856 g / molar mass of acid = 0.0345 moles acid
Since two moles of NaOH are needed for every mole of acid, we can divide the number of moles of NaOH by two to get the number of moles of acid: 15.742 moles NaOH / 2 = 7.871 moles acid
0.0345 moles acid x molar mass of acid = molar mass of acid
molar mass of acid = 0.0345 moles acid x molar mass of acid = 306.325 g/mol
To learn more about molar mass
https://brainly.com/question/837939
#SPJ1
56. What would be the valence electrons of 14Si, 16S, 32Ge respectively?
A. 4, 2,2
B. 4, 6,8
C. 4, 6,6
D. 4,6,4
Answers
Answer: D. 4,6,4
Explanation:
The valence electron is the electron found in the outermost shell of an atom, and participates in bond formation.
For the following element, the valence electron can be noticed when we write the electronic configuration of each element.
Silicon = 1s2 2s2 2P6 3s2 3p2
Sulphur = 1s2 2s2 2P6 3s2 3p4
Germanium = 1s2 2s2 2P6 3s2 3p6 3d10 4s2 4p2
determine the total mass of 1 pentanol that will dissolve in 110 grams of water to produce a saturated solution
Answers
To determine the total mass of 1-pentanol that will dissolve in 110 grams of water to produce a saturated solution, we need to use the concept of solubility and molar mass. What is solubility? Solubility refers to the ability of a solute to dissolve in a solvent. It is measured in units of grams per 100 grams of solvent at a specific temperature.
A solution is said to be saturated if it contains the maximum amount of solute that can dissolve in the solvent at a specific temperature. The solubility of a solute depends on factors such as temperature, pressure, and the nature of the solvent and solute. What is molar mass? Molar mass refers to the mass of one mole of a substance. It is measured in units of grams per mole. The molar mass of a compound can be calculated by adding the atomic masses of the elements in the compound. Explanation: Given data: Mass of water = 110 grams To determine: Total mass of 1-pentanol that will dissolve in 110 grams of water to produce a saturated solution Solution: Firstly, let's find out the solubility of 1-pentanol in water. According to the solubility chart, the solubility of 1-pentanol in water at 25°C is 22.5 g/100 ml (or 22.5 g/110 g).So, we can say that 22.5 g of 1-pentanol can dissolve in 100 g of water to produce a saturated solution at 25°C.Now, we can use the proportionality method to calculate the total mass of 1-pentanol that will dissolve in 110 g of water.22.5 g of 1-pentanol dissolves in 100 g of water. So, x grams of 1-pentanol will dissolve in 110 g of water. x = (22.5 × 110) / 100x = 24.75 g Therefore, the total mass of 1-pentanol that will dissolve in 110 g of water to produce a saturated solution is 24.75 grams.
for more such questions on solubility .
https://brainly.com/question/24561285
#SPJ11
Pls help me I don’t know how to do this

Answers
Explanation:
We have a 63.9 g sample of calcium hydroxide. First we have to convert those grams into moles. To do that we have to use the molar mass of calcium hydroxide.
Calcium hydroxide = Ca(OH)₂
molar mass of Ca = 40.08 g/mol
molar mass of O = 16.00 g/mol
molar mass of H = 1.01 g/mol
molar mass of Ca(OH)₂ = 1 * 40.08 g/mol + 2 * 16.00 g/mol + 2 * 1.01 g/mol
molar mass of Ca(OH)₂ = 74.10 g/mol
mass of Ca(OH)₂ = 63.9 g
moles of Ca(OH)₂ = 63.9 g /(74.10 g/mol)
moles of Ca(OH)₂ = 0.862 moles
In 1 molecule of Ca we have 2 atoms of O. So in 1 mol of Ca(OH)₂ we will have 2 moles of O atoms.
1 mol of Ca(OH)₂ = 2 moles of O atoms
moles of O atoms = 0.862 moles of Ca(OH)₂ * 2 moles of O /1 mol of Ca(OH)₂
moles of O atoms = 1.724 moles
One mol is similar to a dozen. When we say that we need a dozen eggs we know that we need 12 eggs. If we want a mol of eggs, we want 6.022*10^23 eggs. So one mol of something is 6.022 * 10^23 of that.
1 mol of O atoms = 6.022 * 10^23 atoms
n° of O atoms = 1.724 moles * 6.022 * 10^23 atoms/1 mol
n° of O atoms = 1.04 * 10^24 atoms
Answer: In a 63.9 g sample of Ca(OH)₂ we have 1.04 *10^24 atoms of oxygen.
Sodium carbonate decomposes to produce sodium oxide and
carbon dioxide.
Answers
Answer:
air
Explanation:
you breathe ok carbon dioxide
write the correct balanced equation of Lead 2 Oxide and hydrogen
Answers
The balanced equation of the reaction between hydrogen and lead (ii) oxide is given is:
PbO + H₂ ----> Pb + H₂O
Balanced chemical equationA chemical equation is balanced if the number of atoms of each element on the reactants side of the equation is equal to the number of atoms of each element on the product side.
A balanced chemical equation follows the law of conservation of mass that matter is neither created nor destroyedReaction between hydrogen and lead (ii) oxideHydrogen reacts with lead(ii) oxide to produce lead and water
The balanced equation of the reaction between hydrogen and lead (ii) oxide is given is:
PbO + H₂ ----> Pb + H₂O
Learn more about balanced equations at: https://brainly.com/question/15995710
Mrs. Keep burns a walnut under a beaker of water. The beaker contains 100 g of water which warms from 25oC to 30oC. Assuming that all the heat from the burning walnut goes into the water and none of the heat is lost to the air or the beaker, how many calories are in the walnut?
a 2100 calories
b 10,500 calories
c not enough information is given
d 500 calories
Answers
The amount of heat gained by the water is 500 calories. Thus, option D is correct.
Given:
Mass of water (m) = 100 g
Change in temperature (ΔT) = 30°C - 25°C = 5°C
The specific heat capacity of water (c) is approximately 1 calorie/gram°C.
Now, the amount of heat gained by the water,
Q = mcΔT
Where:
Q is the heat gained or lost by the substance
m is the mass of the substance
c is the specific heat capacity of the substance
ΔT is the change in temperature
Plugging in the values into the formula:
Q = 100 × 1 × 5
Q = 500 calories
Therefore, the amount of heat gained by the water is 500 calories.
Learn more about heat, here:
https://brainly.com/question/31608647
#SPJ1
How many moles of NH3 could you produce with 0.24 moles of H2 and excess nitrogen gas?
Answers
Answer:
\(0.16\; \rm mol\).
Explanation:
\(\rm H_2\) and \(\rm N_2\) react to produce \(\rm NH_3\) through the following reaction:
\(\rm 2\; N_2 + 3\; H_2 \to 2\; NH_3\).
The ratio between the coefficients and \(\rm NH_3\) and \(\rm H_2\) is:
\(\displaystyle \frac{n({\rm NH_3})}{n({\rm H_2})} = \frac{2}{3}\).
This coefficient ratio would be the ratio between the quantity of \(\rm NH_3\) produced and the quantity of \(\rm H_2\) consumed in this reaction only if \(\rm H_2\!\) is a limiting reactant.
The other reactant in this reaction, \(\rm N_2\), is in excess. Hence, \(\rm H_2\) would be the limiting reactant. Hence, the coefficient ratio could be used to find the quantity of \(\rm NH_3\) produced in this reaction.
\(\begin{aligned}n({\rm NH_3}) &= \frac{n({\rm NH_3})}{n({\rm H_2})} \cdot n({\rm H_2}) \\ &= \frac{2}{3} \times 0.24\; \rm mol \\ &= 0.16\; \rm mol \end{aligned}\).
The beta-pleated sheet is characterized by orientation of ______ the molecular axis.
(1) H bonds parallel to
(2) H bonds perpendicular to
(3) ionic bonds parallel to
(4) ionic bonds perpendicular to
(5) peptide bonds perpendicular to
Answers
The beta-pleated sheet is characterized by orientation of H bonds perpendicular to the molecular axis. The beta-pleated sheet is a secondary structure of proteins where the peptide chains are arranged in a zigzag manner, with adjacent chains lying in opposite directions.
These chains are held together by hydrogen bonds between the carbonyl oxygen of one chain and the amino hydrogen of an adjacent chain. These hydrogen bonds are oriented perpendicular to the molecular axis. This orientation of the hydrogen bonds in the beta-pleated sheet allows for the formation of a stable and rigid structure. The perpendicular orientation of the hydrogen bonds also creates a pleated appearance, with the peptide chains arranged in alternating upward and downward directions. The beta-pleated sheet is commonly found in proteins involved in structural roles, such as in silk and spider webs.
In beta-pleated sheets, the protein chains run alongside each other, and the hydrogen bonds form between the chains. These hydrogen bonds are perpendicular to the molecular axis, providing stability to the structure. The hydrogen bonds that form between the carbonyl oxygen atom of one amino acid residue and the amide hydrogen atom of another amino acid residue create the beta-pleated sheet structure. This arrangement causes the protein chains to fold in a way that the hydrogen bonds are perpendicular to the molecular axis.
To know more about molecular visit
https://brainly.com/question/14614762
#SPJ11
what is the scientific name for salt
Answers
Answer:
Sodium chloride
Explanation:
What is the scientific term for salt?
To most people, salt refers to table salt, which is sodium chloride. Sodium chloride forms from the ionic bonding of sodium ions and chloride ions.
Glucose is absorbed into the blood in the small intestine. Most of the glucose is absorbed by diffusion. How does the glucose concentration in the blood compare to the glucose concentration in the small intestine? *
Pick one please
The concentration in the blood is higher.
The concentration in the blood is lower.
The concentration in the blood is the same.
None of the above.
Answers
Answer: The concentration in the blood is lower
Explanation:
In which numbered position would a Period 4 element that is a metal with three valence electrons be located?
Answers
Did u get the answer im taking the test rn
Which of the following mixtures would result in the pK, of the acid being obtained from a direct pH
measurement of the resulting solution?
A. 25 cm³ 0.1 mol dm^-3 HCl and 25 cm^3 0.1 mol dm^-3 NaCl
B. 25 cm³ 0.1 mol dm^-3 NaOH and 25 cm³ 0.1 mol dm^-3 CH3COOH
C. 12.5 cm³ 0.1 mol dm^-3 CH3COOH and 25 cm³ 0.1 mol dm^-3 NaOH
D. 12.5 cm³ 0.1 mol dm^-3 NaOH and 25 cm³ 0.1 mol dm^-3 CH3COOH
Answers
The mixtures would result in the pk, of the acid being obtained from a direct pH measurement of the resulting solution is 25 cm³ 0.1 mol dm⁻³HCl and 25 cm³ 0.1 mol dm⁻³ NaCl. Therefore, option A is correct.
What is pH ?The pH scale, which previously stood for "potential of hydrogen," is used to describe how acidic or basic an aqueous solution is. The pH values of acidic solutions are lower than those of basic or alkaline solutions.
The pH scale determines how acidic or basic water is. The range is 0 to 14, with 7 representing neutrality. Acidity is indicated by pH values below 7, whereas baseness is shown by pH values above 7. In reality, pH is a measurement of the proportion of free hydrogen and hydroxyl ions in water.
A solution's pH is a significant indicator of its chemical composition. The pH can affect how readily available nutrients are, how biological processes work, how bacteria behave, and how chemicals behave.
Thus, option A is correct.
To learn more about pH, follow the link;
https://brainly.com/question/15289741
#SPJ1
Hey can someone please help me with this

Answers
Answer:
This is not a redox reaction.
Explanation:
A redox reaction is a reaction that involves reduction and oxidation processes. The best way to identify a redox reaction. Changes in the oxidation number f the species indicates that it is a redox reaction.
Na+
Reactant side = +1, Product Side = +1; No change
Cl-
Reactant side = -1, Product Side = -1; No change
Ag+
Reactant side = +1, Product Side = +1; No change
N
Reactant side = +5, Product Side = +5; No change
O
Reactant side = -2, Product Side = -2; No change
There is no change in any of the oxidation numbers, Hence this is not a redox reaction.
how can you blance it and make it equal on both sides
2H2+o2=2H2o blance it
Answers
Answer:
it have been already balanced
2H2 + O2 = 2H2O.
The size of an atom generally increases down a group and from right to left across a period. up a group and from left to right across a period. down a group and from left to right across a period up a group and from right to left across a period. up a group and diagonally across the Periodic Table. Which set shows the correct resonance structures for SeO_2? SeO_2 does not have a resonance structure. Which of the following ions doesn't have the same electronic configuration noble gas? Cl_- N^3+ S^2- So^3+ None of the above The bond length of 1.27 Angstrom, what is the dipole moment in debayes, if the charges on H and Cl were +1 and -, respectively? 4.79 D 1.63 D 6.08 D 1.08 D None of the above What is the estimation of the delta H (Bond dissociation energy change) for the following gas phase reaction? CHBr_2 + Cl_2 rightarrow CBr_3Cl + HCl D(C-H) = 413kj, D(Cl-Cl) = 242 kJ, D(C-Cl) = 328 kJ, D(H-Cl) = 43kJ.
Answers
Size of an atom increases as we move down a group and from left to right across a period
Define an atom?
An atom is a unit of matter that specifically characterizes a chemical element. One or more negatively charged electrons surround the core nucleus of an atom, which is made up of all of them. One or more protons and neutrons, which are comparatively heavy particles, can be found in the positively charged nucleus.
In a group, as the atomic number rises, the atomic size expands from top to bottom. Valence electrons are located farther from the nucleus because there are more filled energy levels, which increases atomic size.
Atomic size grows as a function of period number, number of shells, and so forth. Since there are more electrons in each shell as we move from left to right in a period, the force of attraction between the nucleus and electrons, which have positive charges, is stronger, bringing the shells closer to the nucleus and shrinking the size of the atom.
To know more about atomic number use link below:
https://brainly.com/question/11353462
#SPJ1
convert 18.9 moles to MgCl2 to formula units
Answers
Answer:
18.9 moles of MgCl2 = 17.834 kg of MgCl2
Explanation:
The molecular weight of MgCl is 80.0 g/mol . So, to convert the given mole amount to grams, multiply this by this number, which is constant for all compounds with a specific composition (mass fraction).
Considering the original question was in the context of chemistry, I wanted to make it seem formal and more educational too. Hopefully that worked!
EDIT: Came up with some text around what happens inside cells that would have made it better if someone just had an issue converting units, but I doubt my answer will be accepted >.<
how many moles and grams of carbon are present in 11.85 g of aspirin CgH804? moles of C mol grams of C X g
Answers
The moles and the grams of carbon are present in 11.85 g of aspirin C₉H₈O₄ is 0.585 mol.
The chemical formula for the compound = C₉H₈O₄
The mass of the aspirin = 11.85 g
The moles of the aspirin = mass / molar mass
The moles of the aspirin = 11.85 / 180
The moles of the aspirin = 0.065 mol
The moles of the carbon in aspirin, C₉H₈O₄ = 9 × 0.065
The moles of the carbon in aspirin, C₉H₈O₄ = 0.585 mol
Thus, the moles of the carbon atom is 0.085 in the aspirin that is C₉H₈O₄ compound .
To learn more about moles here
https://brainly.com/question/1895167
#SPJ4
Which element is in Group 7?
Responses
Chlorine (Cl)
Oxygen (O)
Lithium (Li)
Calcium (Ca)

Answers
The chlorine element is present in group 7.
The elements which has 7 valence electrons , is kept in group 7.
1) Chlorine (Cl):
The atomic number of chlorine is 17 and its electronic configuration is 2,8,7. It has 7 valence electrons. Hence, it will be kept in group 7.
2) Oxygen (O):
The atomic number of oxygen is 8 and its electronic configuration is 2,6.
It has 6 valence electrons. Hence, it will be kept in group 6.
3) Lithium (Li)
The atomic number of chlorine is 3 and its electronic configuration is 2,1. It has 1 valence electrons. Hence, it will be kept in group 1.
4) Calcium (Ca):
The atomic number of calcium is 20 and its electronic configuration is 2,8,8,2. It has 2 valence electrons. Hence, it will be kept in group 2.
Therefore, chlorine element will be kept in group 7.
To know more about element
https://brainly.com/question/1513999
#SPJ1