Answers
The equation for the molarity, or molar concentration, is:
\(C=\frac{n_{\text{solute}}}{V_{\text{solution}}}\)Where C is the molarity, n is the number of moles of solute and V is the volume of solution.
Since we know the molarity and the volume of solution, we can calculate the number of moles of solute:
\(\begin{gathered} V_{\text{solution}}=6.99L \\ C=2.87M=2.87mol\/L \\ n_{\text{solute}}=C\cdot V_{\text{solution}} \\ n_{\text{solute}}=2.87mol\/L\cdot6.99L=20.0613mol\approx20.06mol \end{gathered}\)So, there is approximately 20.06 mol in the solution.
Related Questions
Which of the following statements correctly describe reaction intermediates? Select all that apply.
Multiple select question.
Answers
A) Reaction intermediates are short-lived molecules that form as a result of a chemical reaction.C) Reaction intermediates are molecules that are produced as a result of a chemical reaction.
What is the molecules ?
Molecules are the smallest unit of a substance that can exist independently and has the same chemical properties as the original substance. They are made up of atoms that are connected together by chemical bonds. Molecules are the basic building blocks of all matter, and they exist in a variety of shapes and forms, including solids, liquids, and gases. Molecules can also be composed of multiple elements, such as water which is composed of two hydrogen atoms and one oxygen atom. Molecules can be found in a wide range of materials, from the air we breathe to the food we eat.
To learn more about molecules
https://brainly.com/question/26044300
#SPJ4
Complete question
A) Reaction intermediates are short-lived molecules that form as a result of a chemical reaction.
B) Reaction intermediates are stable molecules that form as a result of a chemical reaction.
C) Reaction intermediates are molecules that are produced as a result of a chemical reaction.
D) Reaction intermediates are molecules that remain unchanged throughout a chemical reaction.
what is the atomic number of an atom woth six valence electrons?
(A) 8
(B)10
(C) 12
Answers
Answer:
8
Explanation:
The atomic mass of an atom refers to the number of protons in the nucleus of an atom. Hence, an element with 6 valence electron mean that it has 6 electrons in its outermost shell.
The atomic Number of elements with 6 valence electrons cannot necessarily be the same, however, elements with 6 valence electrons belong to group six on the periodic table.
From the options given above, :
Atomic number of 8 :
Configuration : 2, 6
Atomic number of 10 :
Configuration : 2, 8
Atomic number of 12 :
Configuration : 2, 8, 2
Hence, only the element with atomic number of 8 has 6 valence electrons in its outer most shell, hence, the answer.
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Label the equivalence point on the graph of pH versus volume of the titration of a strong acid
and strong base shown below.
a. What volume of base was needed to neutralize the acid?
b. What is the pH at the equivalence point?
c. how does the number of moles of hydro i um ions and hydroxide ions compare at the equivalence point?
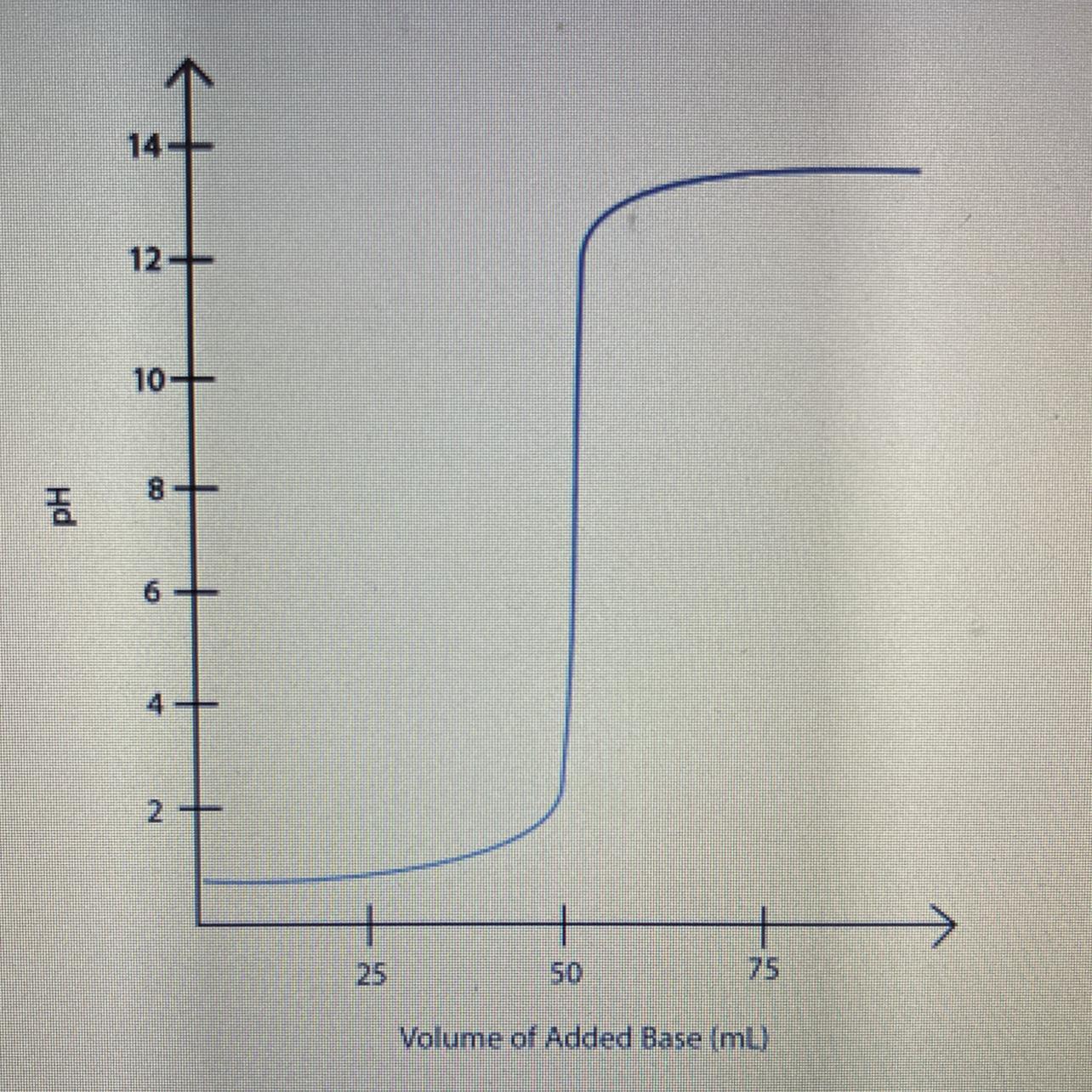
Answers
Based on the titration curve, the titration is of a strong acid strong base and pH at equivalence point is 7.
What is equivalence point?The equivalence point in an acid-base titration is the point at which equal amount of acid and base have reacted.
The equivalence point in a strong acid-strong base titration is at pH 7.
In a weak acid-strong base titration, the pH is greater than 7 at the equivalence point.
In a weak base-strong acid titration, the pH is less than 7 at the equivalence point.
From the titration curve;
volume of base was needed to neutralize the acid is 50 mL pH at the equivalence point is 7the number of moles of hydronium ions and hydroxide ions at the equivalence point are equal.Therefore, the titration curve is of a strong acid strong base.
Learn more about equivalence point at: https://brainly.com/question/24584140
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
Identify any formal charges that are missing from the structures of 1 and 2 [HINT: You should add all missing valence electrons first].

Answers
These are the formal charges for compounds 1 and 2:
1 | N = 0, O = -1, Cl = -12 | N = +1, O = -1, C = 0How to determine formal charges?The formal charge of an atom is the charge that an atom would have if all of the electrons in a bond were shared equally. To calculate the formal charge of an atom, use the following formula:
formal charge = valence electrons - (bond electrons / 2) - non-bonding electrons
In compound 1, the nitrogen atom has 5 valence electrons, 3 bond electrons (one from each of the two C-N bonds), and 0 non-bonding electrons. Therefore, the formal charge on the nitrogen atom is 0.
The oxygen atom in compound 1 has 6 valence electrons, 2 bond electrons (one from each of the two C-O bonds), and 4 non-bonding electrons. Therefore, the formal charge on the oxygen atom is -1.
The chlorine atom in compound 1 has 7 valence electrons, 1 bond electron (from the C-Cl bond), and 6 non-bonding electrons. Therefore, the formal charge on the chlorine atom is -1.
In compound 2, the nitrogen atom has 5 valence electrons, 1 bond electron (from the N-O bond), and 4 non-bonding electrons. Therefore, the formal charge on the nitrogen atom is +1.
The oxygen atom in compound 2 has 6 valence electrons, 2 bond electrons (one from the N-O bond and one from the C=O bond), and 0 non-bonding electrons. Therefore, the formal charge on the oxygen atom is -1.
The carbon atoms in both compounds have 4 valence electrons, 4 bond electrons, and 0 non-bonding electrons. Therefore, the formal charge on the carbon atoms is 0.
Find out more on valence electrons here: https://brainly.com/question/13552988
#SPJ1
what element is this

Answers
Answer:
Nitrogen (N)
Explanation:
From the question given above, the following data were obtained:
Electronic configuration of the element => 1s² 2s²2p³
Next, we shall determine the number of electrons present in the element.
Thus, the number of electrons in the element can be obtained as follow:
Number of electron = 2 + 2 + 3
Number of electron = 7
Next, we shall determine the number of protons in the element.
Since the element is neutral, the number of protons is the same as the number of electrons. Mathematically,
Number of protons = number of electrons
Number of electron = 7
Therefore,
Number of protons = 7
Finally, we shall determine the atomic number of the element.
The atomic number of an element is the number of protons in the atom of the element. Mathematically,
Atomic number = number of protons
Number of protons = 7
Therefore,
Atomic number = 7
Comparing the atomic number of the element ( i.e 7) with those in the periodic table, the name of the element is Nitrogen (N) since no two elements have the same atomic number.
Part B
Follow these steps to complete the table:
1. Reuse the same test tubes from part A, labeled Fe²+ and Fe³+. Be sure they're clean.
2. Add 4 milliliters of iron(II) sulfate to the test tube labeled Fe²+,
3. Add 4 milliliters of iron(III) nitrate to the test tube labeled Fe³+.
4. Add 4 milliliters of potassium iodide to each test tube.
5. Add 1 milliliter of the prepared starch solution to each test-tube.
6. Record your observations, noting any evidence of a chemical reaction. If there is no evidence of a
reaction, write "no reaction."
BIUX² X₂ 10pt
Substances Mixed
iron(II) sulfate, potassium iodide, and starch
iron(III) nitrate, potassium iodide, and starch
Characters used: 191/15000
AVA
Iron Ion Present
iron(II) (Fe²+)
iron(III) (Fe³+)
V
Description of the Reaction
Answers
iron(II) sulfate, potassium iodide, and starch the solution will turn from yellow to blue.
iron(III) nitrate, potassium iodide, and starch will turn from yellow to blue.
Iron sulphate react with potassium iodide and starch the solution which is initially yellow in colour will turn to Blue this is because the fe3+ ion will iron will get reduced to fe2+. This takes place in an acidic solution. Here the iron will be reduced and the iodine will form a complex with starch.
Iron Nitrate react with potassium iodide and starch the solution which is initially yellow in colour will turn to Blue this is because the fe3+ ion will iron will get reduced to fe2+. This takes place in an acidic solution. Here the iron will be reduced and the iodine will form a complex with starch.
Learn more about chemical reactions here https://brainly.com/question/11231920
#SPJ1
The solution will change from yellow to blue in the presence of FeSO4, KI, and starch.
Starch and Fe(NO3)3 will change from yellow to blue.
Starch and KI react with FeSO4 to produce an initially yellow solution that becomes blue. this is due to the fact that in an acidic solution, the iron ion fe3+ is reduced to fe2+. Here, the iron will be reduced and the iodine will combine with the starch to produce a complex because of the reduction of the iron ion, the fe3+ ion, in the Fe(NO3)3 reaction with KI and starch, the solution's original yellow color changes to blue. This occurs in an acidic liquid. Here, the iron will be reduced and the iodine will combine with the starch to produce a complex.To know more about Starch visit : https://brainly.com/question/11444445
#SPJ1
Why did the model of the atom change?
A. New materials became available to build better models.
B. The structure of atoms changed over time.
C. Scientists did not like the original model.
D. Experiments provided new evidence about the atom.
Answers
All forms of energy can exist as either ________ or ________ energy.
Answers
Answer:
potentiol or kenetic
Explanation:
Once the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to
Answers
Once the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to zero.
What is potential energy?Potential energy is a type of energy that is stored and depends upon the relative height of system. If its height is increased, the potential energy is also increases while on the other hand, if the body comes to the surface of the earth, its potential energy will be zero due to no height of the object. We know that potential energy is equals to product of mass, gravity and height of an object.
So we can conclude that if the roller coaster train gets closer to the bottom of the hill, its kinetic energy increases to 1,100 J, and its potential energy decreases to zero.
What volume of CO2(g), measured at STP is produced if 15.2 grams of CaCO(s) is heated?
Answers
Answer:
Volume = 3.4 L
Explanation:
In order to calculate the volume of CO₂ produced when 15.2 g of CaCO₃ is heated, we need to first write out the balanced equation of the thermal decomposition of CaCO₃:
CaCO₃ (s) + [Heat] ⇒ CaO (s) + CO₂ (g)
Now, let's calculate the number of moles in 15.2 g CaCO₃:
mole no. = \(\mathrm{\frac{mass}{molar \ mass}}\)
= \(\frac{15.2}{40.1 + 12 + (16 \times 3)}\)
= 0.1518 moles
From the balanced equation above, we can see that the stoichiometric molar ratios of CaCO₃ and CO₂ are equal. Therefore, the number of moles of CO₂ produced is also 0.1518 moles.
Hence, from the formula for the number of moles of a gas, we can calculate the volume of CO₂:
mole no. = \(\mathrm{\frac{Volume \ in \ L}{22.4}}\)
⇒ \(0.1518 = \mathrm{\frac{Volume}{22.4}}\)
⇒ Volume = 0.1518 × 22.4
= 3.4 L
Therefore, if 15.2 g of CaCO₃ is heated, 3.4 L of CO₂ is produced at STP.
Which of the following is the most likely impact of clearing natural landscape to construct high-rise buildings?
Decrease in pollution
Scarcity of drinking water
Increase in animal population
Abundance of non-native plants
Answers
Answer:
the abundance of non-native plants. this MAY be right.
Explanation:
i thought it would be an increase in pollution.
See the Attached Picture/File
Answers
Answer:
The most important sodium compounds are table salt (NaCl), soda ash (Na2CO3), baking soda (NaHCO3), caustic soda (NaOH), sodium nitrate (NaNO3), di- and tri-sodium phosphates, sodium thiosulfate (Na2S2O. 5H2O), and borax (Na2B4O. 10H2O)
KCIO3 -> KCI + 02
How many moles of KCI are produced if 6743 grams of KCIO3 decomposes?
Answers
55.03 moles of KCI are produced when 6743 grams of \(KClO_{3}\) decomposes
To determine the number of moles of KCl produced when 6743 grams of \(KClO_{3}\) decomposes, we need to use the concept of molar mass and the balanced chemical equation.
First, let's calculate the molar mass of \(KClO_{3}\)
The molar mass of potassium (K) is approximately 39.10 g/mol.
The molar mass of chlorine (Cl) is approximately 35.45 g/mol.
The molar mass of oxygen (O) is approximately 16.00 g/mol.
So, the molar mass of \(KClO_{3}\) is:
(39.10 g/mol) + (35.45 g/mol) + (3 * 16.00 g/mol) = 122.55 g/mol.
Now, we need to calculate the number of moles of \(KClO_{3}\):
Number of moles = Mass / Molar mass
Number of moles = 6743 g / 122.55 g/mol = 55.03 mol.
According to the balanced chemical equation:
2\(KClO_{3}\) -> 2 KCl + 3 O2,
we can see that for every 2 moles of \(KClO_{3}\), we obtain 2 moles of KCl.
Therefore, the number of moles of KCl produced will be equal to the number of moles of \(KClO_{3}\) since the ratio is 1:1. Thus, 55.03 moles of KCl will be produced.
Know more about molar mass here:
https://brainly.com/question/837939
#SPJ11
In addition to hydronium ions which type of ion do acids produce? (Choose all that apply)
A. Anion
B. A negative ion
C. Cation
D.A positive ion
Answers
Answer:
B
Explanation:
i just read it na, but i forgot the explanation
Answer:
A. and B.
Explanation:
Anion is negative as well as a negative ion.
(Just took the quiz)
Convert 100.6 Kelvin to degrees C.
°C = K - 273
[?] °C
Answers
Answer:
-172.6 °C
Explanation:
You want to know the Celsius equivalent of the temperature 100.6 K.
ConversionThe relation is ...
C = K - 273.15
C = 100.6 -273.15 = -172.55
The temperature is -172.55 °C, about -172.6 °C.
__
Additional comment
We have rounded to tenths, because that is precision of the temperature given. If you use 273 as the conversion constant, you will get -172.4.
I need help calculating the error % in molar mass
Answers
Error % = |(experimental - actual) / actual| x 100%
For example, let's say the actual molar mass of a compound is 100 g/mol, and the experimental molar mass determined in the lab is 95 g/mol. The error percentage would be:
Error % = |(95 - 100) / 100| x 100%
Error % = |-0.05| x 100%
Error % = 5%
Therefore, the error percentage in molar mass is 5%
please help me im confused

Answers
The empirical formulas of the four ionic compounds that can be formed from the given ions are NH4CN, Fe(IO3)3, NH4I, and Fe(CN)3.
Empirical formula of ionic compoundsIonic compounds are formed when oppositely charged ions are attracted to each other by electrostatic forces to form a neutral compound. In this case, we have four different ions: NH^+, CN^-, IO3^-, and Fe^3+. To form an ionic compound, we need to pair up ions with opposite charges in such a way that the overall charge of the compound is neutral.
Ammonium cyanide: NH4CNThe ammonium ion (NH4^+) has a charge of +1, and the cyanide ion (CN^-) has a charge of -1. When they combine, the charges cancel out, giving a neutral compound with the formula NH4CN.
Iron(III) iodate: Fe(IO3)3The iron(III) ion (Fe^3+) has a charge of +3, and the iodate ion (IO3^-) has a charge of -1. To balance the charges, we need three iodate ions for every iron(III) ion. Therefore, the formula for the compound is Fe(IO3)3.
Ammonium iodide: NH4IThe ammonium ion (NH4^+) has a charge of +1, and the iodide ion (I^-) has a charge of -1. When they combine, the charges cancel out, giving a neutral compound with the formula NH4I.
Iron(III) cyanide: Fe(CN)3The iron(III) ion (Fe^3+) has a charge of +3, and the cyanide ion (CN^-) has a charge of -1. To balance the charges, we need three cyanide ions for every iron(III) ion. Therefore, the formula for the compound is Fe(CN)3.
More on ionic compounds can be found here: https://brainly.com/question/3222171
#SPJ1
What type of wave travels quickly through solid, liquid, and gas?
A. primary
B. Secondary
C. Love
D. Rayleigh
Answers
Answer: sound waves
Explanation: Of the three mediums (gas, liquid, and solid) sound waves travel the slowest through gases, faster through liquids, and fastest through solid
The gas in a 250.0 mL piston experiences a change in pressure from 1.25 atm to 2.00 atm. What is the new volume (in mL) assuming the moles of gas and temperature are held constant?
Answers
Answer:
The new volume of the gas is 156.25 mL.
Explanation:
According to Boyle's Law, at constant temperature and number of moles, the pressure and volume of a gas are inversely proportional.
So, we can use the following equation to solve for the new volume (V2):
P1V1 = P2V2
Where P1 and V1 are the initial pressure and volume, and P2 and V2 are the final pressure and volume, respectively.
Substituting the given values:
(1.25 atm) x (250.0 mL) = (2.00 atm) x V2
Solving for V2:
V2 = (1.25 atm x 250.0 mL) / (2.00 atm)
V2 = 156.25 mL
Therefore, the new volume of the gas is 156.25 mL.
How many grams are in 3.1 moles of H2SO4?
Answers
Answer:
98.07848 grams.
Explanation:
to an atom.
Neutrons add only
a. mass
b.ions
C. positive charge
d. negative charge
Please select the best answer from the choices provided
A
В
С
D
Pls help fast due in 10 mins!!! :(((((((( ughhh please!
Answers
Answer:
The answer is A, Neutron adds only mass
A balloon has a volume of 0.8.0 m3. As it rises in the earth s atmosphere, its volume expands. What will be its new volume if its original temperature and pressure are 20 C and 1.0 atm, and its final temperature and pressure are -40 C and 0.10 atm
Answers
Answer:
6.36 m³
Explanation:
Using the general gas law
P₁V₁/T₁ = P₂V₂/T₂
Given that P₁ = 1.0 atm, V₁ = 0.8 m³ and T₁ = 20 °C = 273 + 20 = 293 K and P₂ = 0.10 atm, V₂ = unknown and T₂ = -40 °C = 273 + (-40) = 273 - 40 = 233 K
So, V₂ = P₁V₁T₂/P₂T₁
Substituting the values of the variables into the equation, we have
V₂ = 1.0 atm × 0.8 m³ × 233 K/0.10 atm × 293 K
V₂ = 186.4 atm-K-m³/29.3 atm-K
V₂ = 6.36 m³
So, the new volume of the balloon is 6.36 m³.
5. The Santa Ana winds, also known as "devil winds", are strong and dry high-pressure masses of air that begin in the Great Basin mountains and then affect coastal areas in Southern California. Assume that these winds consists of a mixture of nitrogen and oxygen gases that behave ideally and that they begin at a pressure and temperature of 0.705 atm of 22.0 0C, respectively, and that this mass of air is then adiabatically and reversibly compressed to a pressure of 1.00 atm when it descends to the coastal regions. Calculate the final temperature.
Answers
Answer:
Explanation:
Given that;
The initial pressure = 0.705 atm
The initial temperature = 22.0° C
The final pressure = 1.00 atm
The objective is to calculate the final temperature,
Since the change occurs adiabatically and reversibly, the final temperature can be calculated by using the thermodynamics equation:
\((\dfrac{T_2}{T_1})^r= (\dfrac{P_2}{P_1})^{r-1}\)
where;
r = 7/5 = 1.4
\((\dfrac{T_2}{22.0^0})^{1.4}= (\dfrac{1.00}{0.705})^{1.4-1}\)
\((\dfrac{T_2}{22.0^0})^{1.4}= (1.418)^{0.4}\)
\(T_2 = 22 \times 1.418 ^{^{\frac{0.4}{1.4}}\)
\(T_2 = 22 \times 1.418 ^{{0.286}\)
\(T_2 =24.31^0 \ C\)
Question 5
1 pts
What is the correct interpretation of an 85% yield for a given reaction?
85% of the anticipated amount of products were produced (relative to the theoretical yield)
O 15% of the reactants were used up
O 85% of the reactants were used up
15% of the anticipated amount of products were produced (relative to the theoretical yield)
Question 6
1 pts
True or false: The limiting reactant is the reactant that is used up after the reaction is complete.
O True
False
Answers
Answer: a) 85% of the reactants were used up
b) True
Explanation:
Percentage yield is defined as the ratio of actual yield to theoretical yield in terms of percentage. The actual yield is the amount of product obtained after the experiment and the theoretical yield is the amount of ideal product that should have been obtained.
Formula :
\(\text {percentage Yield}=\frac{\text {Actual Yield}}{\text {Theoretical Yield}}\times 100\)
Thus 85 % yield means 85 % of the reactants were used up to give only 85% of products.
The limiting reactant is the reactant that limits the amount of product formed and hence is completely used up after the reaction is complete. Thus the given statement is True.
Giving brainliest!!!!!!!!!!!!!
Answers
I am the first one to comment, please give me the brainliest <3
Airflow through a long 0.2m square air conditioning duct maintained the outer duct surface temperature at 10degree.if the horizontal duct is uninsulated and exposed to air at 35degree in the crawl space beneath at home.
What is the heat gain per unit length of the duct.
Answers
Answer:
125°C/m
Explanation:
The difference in temperature will be:
35 - 10 = 25
The outer duct surface is heated 25°C by the air from 10°C to 35°C;
This heat gain is simply divided by 0.2m to get heat gain per unit length:
25/0.2 = 125
125°C/m
If you're American, this will be °F I believe, so the answer will be:
125°F/m
Determine the theoretical yield, limiting reactant when 0.50 g of Cr and 0.75 g of H3PO4 react according to the following chemical equation?
2Cr + 2 H3PO4 --> 2CrPO4 + 3H2
Answers
The theoretical yield of the reaction is 1.13 g. The acid is the limiting reactant.
What is the theoretical yield?In a given chemical reaction, the theoretical yield can only be obtained from the balanced reaction equation. We have been given the balanced reaction equation in the question so we can work from there.
Number of moles of Cr = 0.50 g /52 g/mol = 9.6 * 10^-3 moles
Number of moles of acid = 0.75 g/98 g/mol = 7.7 * 10^-3 moles
Given that the reaction is 1:1, the limiting reactant would be the acid.
The theoretical yield is obtained from;
Number of moles of product * molar mass of product
We substitute to obtain;
7.7 * 10^-3 moles * 147 g/mole (since the reaction is 1:1)
= 1.13 g
Learn more about theoretical yield:https://brainly.com/question/14966377
#SPJ1
Which of the following does NOT describe elements? can join together to form compounds all the particles are alike can be broken down into simpler substances have unique sets of properties
Answers
Answer:
Can be broken down into simpler substances.
Explanation:
Elements are made of atoms, and that is the simplest understood unit of the universe. :) nOw GiB mY 50000 points.
Answer:
Can be broken down into simpler substances
Explanation:
Because atoms cannot be created or destroyed in a chemical reaction