antimony pentafluoride, sbf5, reacts with xef4 and xef6 to form ionic compounds, xef3 sbf6- and xef5 sbf6-. describe the molecular geometries of the cations and anion in these two compounds (include angles).
Answers
XeF3+ has a linear molecular geometry with bond angles of approximately 180 degrees.
XeF5+ has a square pyramidal molecular geometry with bond angles of approximately 90 degrees and 120 degrees.
SbF6- has an octahedral molecular geometry with bond angles of approximately 90 degrees.
In the compounds XeF3SbF6- and XeF5SbF6-, the cations are XeF3+ and XeF5+, respectively, and the anion is SbF6-. Let's describe the molecular geometries of these species:
XeF3+ (Xenon Trifluoride Cation):
XeF3+ has a linear molecular geometry. It consists of a central xenon atom (Xe) bonded to three fluorine atoms (F) in a linear arrangement. The bond angles in XeF3+ are approximately 180 degrees.
XeF5+ (Xenon Pentafluoride Cation):
XeF5+ has a square pyramidal molecular geometry. It consists of a central xenon atom (Xe) bonded to five fluorine atoms (F). The geometry is best described as a square base with an additional fluorine atom above the plane, giving it a pyramidal shape. The bond angles in XeF5+ are approximately 90 degrees between the axial fluorine atoms and 120 degrees between the equatorial fluorine atoms.
SbF6- (Hexafluoroantimonate Anion):
SbF6- has an octahedral molecular geometry. It consists of a central antimony atom (Sb) bonded to six fluorine atoms (F). The arrangement of the fluorine atoms around the central antimony atom is similar to the six faces of an octahedron. The bond angles in SbF6- are approximately 90 degrees.
It's important to note that the molecular geometries described here are based on the VSEPR (Valence Shell Electron Pair Repulsion) theory, which predicts the molecular shape based on the repulsion between electron pairs around the central atom.
Learn more about VSEPR from the link given below.
https://brainly.com/question/29755556
#SPJ4
Related Questions
The initial rate of the reaction: BrO3- (aq) + 5Br-(aq) + 8H+(aq) 3Br2(l) + H2O(l) has been measured at the reaction concentrations shown in mol/L. Experiment[BrO3-][Br-][H+]Initial rate (mol/(L∙s)10.100.100.108.0 x 10-420.200.100.101.6 x 10-330.100.200.101.6 x 10-340.100.100.203.2 x 10-3Determine the order of reaction with respect to each reactant
Answers
To determine the order of the reaction with respect to each reactant, we must compare how the reaction rate changes when its concentration changes.
We have in experiment one the same concentration of reactants and a speed equal to 8.0x10^-4 mol/(L.s).
Now, in the second experiment, the concentration of BrO3 doubles, and the rest of the reagents remain the same. The speed is also doubled since 8.0x10^-4 x 2 = 1.6 x 10^-3.
The same happens with Br-, in the third experiment. The rate doubles as the Br concentration doubles.
So for these two reactants, the rate of reaction will be first-order, since as the concentration increases the rate increases in the same proportion.
Now, for H+ we have that by doubling the concentration the rate quadruples. This means that the reaction order is second order. When changing the concentration, the speed changes in order equal to 2
Answer:
the order of reaction will be:
Respect BrO3-: First order
Respect Br-: First order
Respect H+: Second order
If 25.6 mL of a 2.0 M hydroiodic acid solution was used
to make 1000. mL of a dilute solution:
a) How much water was necessary for the dilution?
b) What is the concentration of the dilute hydroiodic acid solution?
i) Based on the calculated concentration, calculate the
pH, [H3O*], [OH-], and pOH of the diluted HI solution.
Answers
a) 974.4 mL of water is necessary for the dilution.
b) i) the diluted hydroiodic acid solution has a concentration of 0.0512 M, a pH is 1.29, an [\(H_{3}O+\)] concentration of 0.0512 M, an [OH-] concentration of 1.27 x \(10^{-13}\) M, and a pOH of 12.71.
a) To calculate the amount of water necessary for the dilution, we need to consider that the volume of the dilute solution is 1000 mL, and we started with 25.6 mL of the concentrated hydroiodic acid solution. Therefore, the amount of water added is the difference between these two volumes:
Volume of water = Volume of dilute solution - Volume of hydroiodic acid solution
Volume of water = 1000 mL - 25.6 mL
Volume of water = 974.4 mL
Therefore, 974.4 mL of water is necessary for the dilution.
b) The concentration of the dilute hydroiodic acid solution can be calculated using the dilution formula:
C1V1 = C2V2
Where C1 is the initial concentration, V1 is the initial volume, C2 is the final concentration, and V2 is the final volume.
In this case, C1 = 2.0 M, V1 = 25.6 mL, C2 = ?, and V2 = 1000 mL.
By substituting the known values into the formula and solving for C2, we get:
(2.0 M)(25.6 mL) = C2(1000 mL)
C2 = (2.0 M)(25.6 mL) / 1000 mL
C2 = 0.0512 M
Therefore, the concentration of the dilute hydroiodic acid solution is 0.0512 M.
i) Based on the calculated concentration, the pH, [\(H_{3}O+\)], [OH-], and pOH of the diluted HI solution can be determined. Since hydroiodic acid is a strong acid, it completely dissociates in water to produce \(H_{3}O+\) ions. Therefore, the concentration of \(H_{3}O+\) ions in the solution is 0.0512 M.
The pH of a solution can be calculated using the equation:
pH = -log[\(H_{3}O+\)]
pH = -log(0.0512) ≈ 1.29
Since hydroiodic acid is a strong acid, the concentration of OH- ions can be considered negligible. Therefore, the pOH can be calculated using the equation:
pOH = 14 - pH
pOH = 14 - 1.29 ≈ 12.71
Finally, the [OH-] concentration can be calculated using the equation:
[OH-] = \(10^{-pOH}\)
[OH-] = \(10^{-12.71}\) ≈ 1.27 x \(10^{-13}\) M
In summary, the diluted hydroiodic acid solution has a concentration of 0.0512 M, a pH of approximately 1.29, an [\(H_{3}O+\)] concentration of 0.0512 M, an [OH-] concentration of approximately 1.27 x \(10^{-13}\) M, and a pOH of approximately 12.71.
Know more about Dilute solution here:
https://brainly.com/question/1615979
#SPJ8
please help me with this

Answers
Answer:
It is A, the answer is A
Explanation:
If only 44 grams of COz are produced, what is the % yield? -
Answers
Answer:
To calculate the percent yield, you need to know the theoretical yield, which is the maximum amount of product that could be produced based on the starting materials and reaction conditions, and the actual yield, which is the amount of product that was actually obtained in the experiment. The percent yield is then calculated using the following formula:
Percent Yield = (Actual Yield ÷ Theoretical Yield) x 100%
In this case, we don't have information about the theoretical yield or the reaction conditions, but we know that only 44 grams of CO2 were produced. Without additional information, we cannot calculate the percent yield. The theoretical yield could be more or less than 44 grams, and the actual yield could also be more or less than the theoretical yield.
Therefore, we need more information to determine the percent yield.
A photon of 498nm was emmited from a silicon atom. Calculate the energy of all the atomic levels of silicon and show that The atomic levels are quantized
Answers
Answer:
3.99 × 10 ^-28
Explanation:
In the equation of E = hc/λ, the h = 6.626 × 10^−34 J and c = 3 × 10^8 m/s
E = 6.626 × 10^−34 × 3 × 10^8 divided by 498
E = 3.99 × 10 ^-28
Select the ion. HCl H 3O +1 H 2O
Answers
Answer:
\(H _{3}O {}^{ + } \)
Hydroxonium ion
The molar mass of carbon monoxide, CO, is 28.0 g/mol. Find the mass in grams of one molecule of CO.
Answers
The molar mass of a substance is defined as the mass of one mole of that substance, which is equal to Avogadro's number of particles. In this case, the molar mass of CO (carbon monoxide) is 28.0 g/mol.
To find the mass of one molecule of CO, we need to calculate the molar mass of CO in atomic mass units (amu). One amu is defined as 1/12 the mass of a carbon-12 atom. The atomic mass of carbon is 12.0107 amu and the atomic mass of oxygen is 15.999 amu. Hence, the molar mass of CO in amu can be calculated as:
Molar mass of CO (amu) = 12.0107 amu + 15.999 amu = 28.00077 amu
Therefore, the mass of one molecule of CO in grams is given by:
Mass of one molecule of CO (g) = Molar mass of CO (amu) * (1 amu) / (6.022 x 10^23 particles/mol)
= 28.00077 amu * (1.66 x 10^-24 g/amu)
= approximately 4.65 x 10^-23 g/particle.
So, one molecule of CO has a mass of 4.65 x 10^-23 grams.
Here you can learn more about molar mass
https://brainly.com/question/10454993#
#SPJ11
What mass of potassium is in a sample of potassium carbonate that contains 13.9 g of oxygen?
Answers
The mass of potassium in the sample of potassium carbonate is 10.5 g.
Potassium carbonate consists of potassium (K), carbon (C), and oxygen (O). To determine the mass of potassium in the sample, we need to use the molar ratio between potassium and oxygen in potassium carbonate.
The molar mass of potassium carbonate is calculated as follows:
K2CO3 = 2(K) + 1(C) + 3(O) = 138.21 g/mol
From the formula, we can see that there are two potassium atoms for every one molecule of potassium carbonate. Therefore, we need to consider the molar mass of potassium (39.10 g/mol) and the molar mass of potassium carbonate to find the mass of potassium in the sample.
Using the molar ratio between potassium and oxygen, we can set up the following proportion:
(2 mol K / 1 mol K2CO3) = (x g K / 13.9 g O)
Solving for x, the mass of potassium, we get:
x = (2 mol K / 1 mol K2CO3) * (39.10 g K / 2 mol K) * (13.9 g O / 16.00 g O)
x ≈ 10.5 g
Therefore, the mass of potassium in the sample of potassium carbonate is approximately 10.5 grams.
Learn more about : Potassium carbonate.
brainly.com/question/28923802
#SPJ11
Given the following chemical reaction: 2H2(g) + O2(g) → 2H2O(l) What is the stoichiometric mixture?
Answers
2 moles of H2 react with 1 mole of O2 gas, according to the balanced reaction. the stoichiometric ratio of the moles of H2 to the moles of O2 is 2:1 as a result.
How can you determine whether a reaction is stoichiometric?When all of the reactants are consumed and none are left after the chemical reaction has finished, it is said to have occurred in a stoichiometric chemical reaction.
What does stoichiometry refer to?Stoichiometry can be summed up as the process of calculating the products and reactants of a chemical reaction. It primarily has to do with numbers. Stoichiometry is a fundamental concept in chemistry that makes it easier to use balanced chemical equations to ascertain the amounts of reactants and products.
To know more about stoichiometry visit:-
https://brainly.com/question/9743981
#SPJ13
Charcoal found under a stone at Stonehenge, England, has a carbon-14 activity that is 0.60 that of new wood. How old is the charcoal
Answers
Charcoal with a carbon-14 activity of 0.60 compared to new wood has less than 5,730 years.
What is a radioactive isotope?A radioactive isotope is an element in nature that emit radioactivity in a given period of time (e.g., the half-life for C14 is equal to 5,730 years).
Radioactive dating is a technique to measure the age of an element by measuring its radioactive activity.
In conclusion, charcoal with a carbon-14 activity of 0.60 compared to new wood has less than 5,730 yr.
Learn more about radioactive dating here:
https://brainly.com/question/8831242
#SPJ1
What makes the atomic radius change along a period in the periodic table?
A. More electrons in the valence shell make the radius bigger.
B. More protons in the nucleus pull the electrons in, making the
atomic radius smaller.
C. The increased atomic mass makes the atomic radius bigger.
D. More electrons pair in orbitals, making the atomic radius smaller.
Answers
Answer:B
Explanation:
which of these conditions results from hyperventilation? group of answer choices decreased alveolar pco2 and increased alveolar po2 increased alveolar pco2 and decreased alveolar po2 an increase in both alveolar pco2 and po2 a decrease in both alveolar pco2 and po2 the same alveolar pco2 as under normal conditions
Answers
Decreased alveolar PCO2 and increased alveolar PO2. When the amount and rate of carbon dioxide exhaled from the lungs exceeds the amount of carbon dioxide the body produces, hyperventilation results.
Hyperventilation may be brought on by emotional stress, worry, or fear. The body perceives itself as being under danger in any of these circumstances, triggering the fight-or-flight response, which causes the body to get ready to act and stay vigilant in order to survive. There is no actual threat in anxiety or emotional tension, but the mind perceives a situation as such and activates the aforementioned response, which results in hyperventilation because taking more breaths results in a lower concentration of carbon dioxide in the blood, which causes physical symptoms.
To know more about hyperventilation visit
https://brainly.com/question/17352284
#SPJ4
Differences between non-biodegradable and biodegradable.
Answers
Answer:
(simple differences)Biodegradable substances are those that degrades or break down naturally...
Non-biodegradable substances are those that do not degrades easily...
Answer:
Biodegradable substances are those that degrades or break down naturally
Non-biodegradable substances are those that do not degrades easily
Which equation represents a decomposition reaction?
O 4P +502 → 2P205
O H2SO4 + 2NaOH → Na2SO4 + 2H2O
O 2C8H18 + 2502 → 16CO2 + 18H2O
O 6NaNO3 → 6NaNO2 + 302
Answers
first and third reaction are considered combustion reaction (reacting with oxygen)
second reaction is acid-base reaction
There are various type of chemical reactions in chemistry like combination reaction , displacement reaction. One of these is the decomposition reaction. The correct option is option D.
What is decomposition reaction?Decomposition reaction is the one in which molecules breaks down in its constituent in presence of energy. Energy can be in any form, it can be light energy , heat energy.
Decomposition of water is done by providing electricity and the reaction is called electrolysis. Lysis means to break. To give decomposition reaction molecule must have more than one element.
The fourth reaction describe a decomposition reaction (NaNO3 decomposed to become NaNO2 and O2). First and third reaction are considered combustion reaction (reacting with oxygen). Second reaction is acid-base reaction.
Therefore, the correct option is option D.
To learn more about decomposition reaction, here:
https://brainly.com/question/13134296
#SPJ2
.
According to the theories of Travis Hirschi and Harold Grasmick, what kind of crime would a person with low self-control be MOST likely to commit?
designing a pyramid scheme to sell to others
stealing a bike that has been left outside
slowly poisoning someone they don’t like at work
planning to break into a well-guarded art gallery
Answers
Answer:
Stealing a bike
Explanation:
Low self-control results in spontaneity in decision-making. It is not a trait you would find in a good planner.
Answer:
stealing a bike that has been left outside
Explanation:
Bike theft is a stealth crime done quickly, and so it's hard to catch someone in the act of stealing. Unlike other crimes, victims don't usually know the criminals who targeted them, so it is harder to start an investigation. When bike thieves are caught, proof of ownership is a real issue.
Took the quiz :)
What is the chemical p2?
Answers
The chemical P2 refers to the diphosphorus molecule, which is a compound made up of two phosphorus atoms bonded together.
Diphosphorus, also known as P2, is a chemical compound composed of two phosphorus atoms covalently bonded together. It is a highly reactive and unstable molecule that is rarely encountered in its pure form. Diphosphorus is an allotrope of phosphorus, meaning it is a different structural form of the same element.
At room temperature, diphosphorus exists as a colorless, odorless gas with a molecular weight of 60.98 g/mol. It is highly reactive and can easily ignite when exposed to air, making it a fire and explosion hazard. Diphosphorus is also highly toxic and can cause severe burns and respiratory problems if inhaled.
Diphosphorus has a unique electronic structure with a triple bond between the two phosphorus atoms. This triple bond makes the molecule highly reactive and unstable, as it seeks to break apart and form more stable bonds with other atoms. As a result, diphosphorus is a useful starting material for the synthesis of other phosphorus-containing compounds.
To know more about Diphosphorus here:
https://brainly.com/question/28024394#
#SPJ11
How many moles do you have if you have 144 L of a gas at SATP?

Answers
Answer
moles = 5.81 mol
Explanation
Given:
Volume = 144 L
AT SATP
1 mole = 24.4651 L
Solution:
1 mole = 24.4651 L
x mole = 144 L
x = 144/24.4651
x = 5.8 mol
How many grams are in 9.05 x 1023 atoms of silicon
Answers
Answer:
42.2075 grams
Explanation:
research the composition of both compact and spongy bone and describe your findings. note the minerals and proteins that make up this tissue.
Answers
Compact Bone is composed of mineralized matrix and bone cells. whereas, Spongy Bone consists of a network of trabeculae, which are thin bony spicules or plates interconnected to create a porous framework.
Compact Bone:
Compact bone, also known as cortical bone, forms the outer layer of bone and provides strength and support. It is composed of mineralized matrix and bone cells.The main minerals found in compact bone include hydroxyapatite, which is a crystalline form of calcium phosphate, and calcium carbonate. These minerals contribute to the hardness and rigidity of the bone tissue.
In addition to minerals, compact bone contains several proteins that contribute to its structure and function. Collagen is the predominant protein found in the bone matrix. It provides flexibility and tensile strength to the bone, allowing it to resist breaking under stress. Other proteins, such as osteocalcin and osteopontin, are involved in mineralization processes and regulate bone remodeling.
Spongy Bone:
Spongy bone, also called trabecular or cancellous bone, is found at the inner layer of bone and forms a lattice-like structure. It consists of a network of trabeculae, which are thin bony spicules or plates interconnected to create a porous framework. This arrangement provides strength to the bone while keeping it lightweight.
Similar to compact bone, spongy bone contains mineralized matrix and bone cells. The minerals present in spongy bone are also hydroxyapatite and calcium carbonate. However, spongy bone has a higher proportion of spaces within its structure compared to compact bone.
Proteins found in spongy bone include collagen, which provides structural support, and other non-collagenous proteins involved in bone development, remodeling, and mineralization.
Overall, both compact and spongy bone consist of mineralized matrix containing hydroxyapatite and calcium carbonate, along with collagen and other proteins that contribute to the structure, strength, and function of the bone tissue. The specific arrangement and density of these components differ between compact and spongy bone, allowing them to fulfill different roles within the skeletal system.
Learn more about Compact Bone at: https://brainly.com/question/2099742
#SPJ11
Bones are made up of two types of tissue: compact and spongy bone. Compact bone (cortical) forms the hard external layer of all bones while spongy (cancellous) bone forms the inner layer of all bones. Both types of bones are composed of specialized cells, mineral salts, and collagen fibers.
Explanation:Our bones are made up of two types of tissue: compact bone and spongy bone. Compact bone, also known as cortical bone, forms the hard external layer of all bones and surrounds the medullary cavity, or bone marrow. This bone tissue consists of units called osteons or Haversian systems, featuring mineral matrix and living osteocytes connected by canaliculi, which transport blood.
Spongy bone, on the other hand, or cancellous bone, forms the inner layer of all bones. Unlike compact bone tissue, spongy bone tissue does not contain osteons. It consists of trabeculae: lamellae that are arranged like rods or plates. In between these trabeculae, we'll find the red bone marrow that is responsible for forming blood cells.
Both types of bone tissues contain specialized cells, mineral salts (mainly calcium and phosphorus), and collagen fibers. The integration of these minerals and proteins is critical for the robust structure and resilience of the skeletal system.
Learn more about Bone Composition here:https://brainly.com/question/34187890
Calculate the pOH of a solution at 25 °C that contains 1.94 x 10^-10 M hydronium ions.
Answers
The pOH of the solution at 25 °C that contains 1.94 x 10^-10 M hydronium ions is 4.29.
To calculate the pOH of a solution at 25 °C that contains 1.94 x 10^-10 M hydronium ions, we need to use the formula:
pOH = -log[OH^-]
To find the [OH^-] concentration, we can use the fact that the product of [H3O^+] and [OH^-] is always equal to 1.0 x 10^-14 at 25 °C (known as the ion product constant, Kw).
[H3O^+][OH^-] = 1.0 x 10^-14
Since we know the [H3O^+] concentration (1.94 x 10^-10 M), we can rearrange the equation to solve for [OH^-]:
[OH^-] = 1.0 x 10^-14 / [H3O^+]
[OH^-] = 1.0 x 10^-14 / 1.94 x 10^-10
[OH^-] = 5.154 x 10^-5 M
Now that we have the [OH^-] concentration, we can use the formula for pOH to calculate the value:
pOH = -log[OH^-]
pOH = -log(5.154 x 10^-5)
pOH = 4.29
Therefore, the pOH of the solution at 25 °C that contains 1.94 x 10^-10 M hydronium ions is 4.29.
For more such questions on hydronium ions
https://brainly.com/question/27586350
#SPJ11
the classic red wine of bordeaux cannot be made from which grape?
Answers
Answer:
Pinot Noir
Explanation:
Cabernet Sauvignon, Merlot, Cabernet Franc, Petit Verdot, Malbec, and on occasion Carménère are the six Bordeaux varietals. The classic red wine of bordeaux cannot be made from pinot noir.
What grape is Bordeaux wine ?Cabernet Sauvignon, Merlot, Cabernet Franc, Petit Verdot, Malbec, and on occasion Carménère are the six Bordeaux varietals. These grapes, which each contribute distinctive qualities to their wines, are combined in various ways to create Bordeaux blends.
A variety of grapes are often used to make red Bordeaux. Cabernet Sauvignon, Cabernet Franc, Merlot, Petit Verdot, Malbec, and Carménère are permitted varietals. The fifth generation Bordeaux wine Château Clerc Milon is one of the few remaining producers of Carménère in today's world.
Cabernet Sauvignon, Merlot, and Cabernet Franc make up the majority of a red Bordeaux mix, with minor amounts of Malbec and Petit Verdo.
Thus, The classic red wine of bordeaux cannot be made from pinot noir.
Learn more about Bordeaux wine follow the link;
https://brainly.com/question/14018645
#SPJ12
Brakes are to a car as
to a nuclear-fission chain reaction.
A. fuel rods are
B. proliferation is
C. free neutrons are
D. control rods are

Answers
Answer:
D. control rods are
I hope dis helps :3
Brakers are to a car as control rods are to a nuclear-fission chain reaction. The correct option is D.
What is a nuclear-fission chain?A chain reaction occurs when neutrons generated during fission cause subsequent fission in at least one more nucleus.
This nucleus creates neutrons, and the cycle continues.
The process can be either controlled (nuclear power) or uncontrolled (nuclear weapons) (nuclear weapons).
Thus, the correct option is D. control rods are.
Learn more about nuclear-fission chain
https://brainly.com/question/24297620
Name the chemical family to which each of the following
elements belongs. KZU
(a) chlorine, CI
(b) magnesium, Mg
(c) potassium, K
(d) helium, He
Answers
d. helium, he
it's medical
b) Alkaline Earth Metal
c) Alkali Metal
d) Noble Gas
When Newton started studying gravity, the concept of gravity was already in place.
A. True
B. False
Answers
Which of the following represents a double-displacement reaction?
a. ABC ➡️AB +C
b. A+B ➡️AB
c. AB + CD➡️AD + CB
d. A + BC ➡️AC + B
Answers
AX + BY —> AY + BX
The first letter of the first letter of the 1st reactant and the last letter of the 2nd reactant go together to make a new product. The second letter of the 2nd reactant and the second letter 1st reactant go together.
11. Group 17 elements are called.
Answers
there is no group 17
in each box, write the number of 's that each labeled carbon has. for example, if the carbon next to the letter a has 's write in the box marked a.
Answers
a = 0, b = 0 and c= 1
Carbon a has 3 bonds with carbon and no hydrogen, carbon b has 4 bonds with carbon and no hydrogen and carbon c has only 1 bond with carbon and 3 bonds with hydrogen
What is carbon?
Carbon is a chemical element with symbol C and atomic mass 6.
It is a chemical building block of all the life on earth. It is widely distributed forming organic compounds when combined with hydrogen, oxygen, nitrogen etc.
Carbon can easily form bond with hydrogen and oxygen atoms
Thus, a = 0, b = 0 and c= 1
Carbon a has 3 bonds with carbon and no hydrogen, carbon b has 4 bonds with carbon and no hydrogen and carbon c has only 1 bond with carbon and 3 bonds with hydrogen.
To learn more about carbon, refer to the link below:
https://brainly.com/question/13719781
#SPJ4
Your question is incomplete, but probably your complete question was
in each box, write the number of H's that each labeled carbon has. for example, if the carbon next to the letter a has 3H's write in the box marked a.
The image is attached

I just want u to check if it’s correct or not no need for explanations
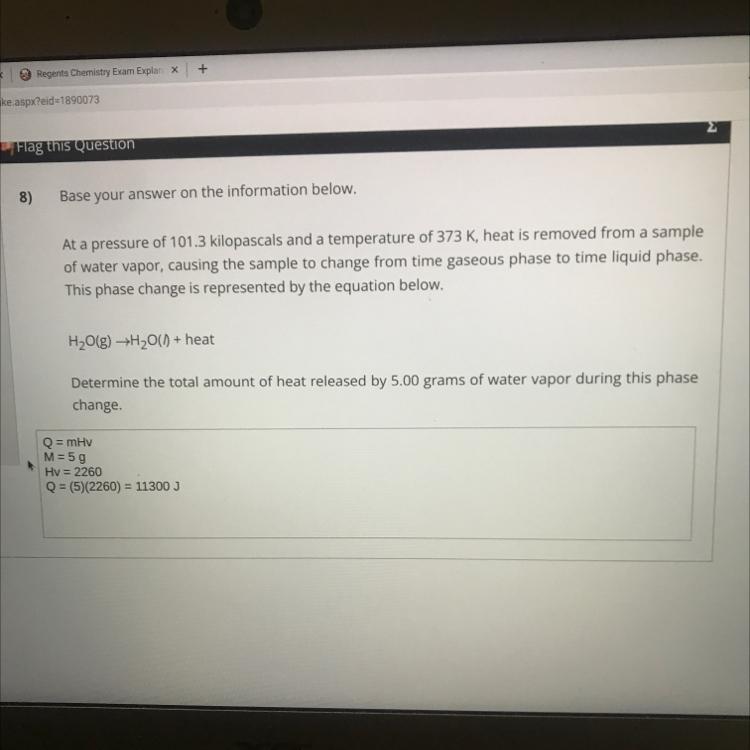
Answers
Given mass = 5g
Heat of vapour = 2260
Heat released during conversion of steam = m * C = 5 * 2260
= 11300J
Your calculations are correct.
State the name of the ion which is oxidised in the following half equations. Cathode: Na+ + e– → Na Anode: 2Cl– → Cl2 + 2e–
Answers
Answer:
hahahahhahhhahahaha
Explanation:
haahahahahhahahhaha
What is the electron configuration for germanium (Ge)?
Answers
Answer:
[Ar] 3d¹⁰ 4s² 4p²
Explanation: