Name the major product of the monobromination of nitrobenzene Name the compound using the appropriate prefix to describe the location off the subsitutents
Answers
The monobromination of nitrobenzene produces para-bromo nitrobenzene as a major product.
The prefix para is used to describe the location of the substituents because para means ‘beside’ or ‘next to’ and indicates that the substituents are positioned on opposite sides of the benzene ring.
Nitrobenzene is an aromatic compound made up of a benzene ring with a nitro group attached to it. Monobromination is an organic reaction whereby a single bromine atom is added to an organic compound. In the case of nitrobenzene, an electrophilic substitution reaction takes place.
This involves an electrophile, such as bromine, attacking the aromatic ring and displacing one of the nitro groups. The bromine atom is then added to the end of the ring, producing the para-bromo nitrobenzene.
know more about benzene ring here
https://brainly.com/question/31490176#
#SPJ11
Related Questions
In a polymer polymer composite , the weight percentage of the polymer 1 and polymer 2 are 20% and 80%. The volume fraction for polymer 1 is 0.5. What is the ratio of the density if the polymer 1 to polymer2?
Answers
Answer:
In a polymer-polymer composite, with a weight percentage of 20% for polymer 1 and 80% for polymer 2, and a volume fraction of 0.5 for polymer 1, we can determine the ratio of their densities.
Explanation:
To calculate the density ratio, we use the formula (0.2 * ρ2) / (0.8 * ρ1), where ρ1 represents the density of polymer 1 and ρ2 represents the density of polymer 2.
This ratio provides a measure of how the densities of the two polymers compare to each other in the composite.
By considering the weight percentage and volume fraction, we can analyze the relative contributions and densities of polymer 1 and polymer 2.
Read more about Polymer composite.
https://brainly.com/question/31392642
#SPJ11
The ratio of the density of polymer 1 to polymer 2 is 0.25. This ratio is determined by the weight percentages and volume fractions of the polymers in the composite.
In a polymer composite, the weight percentage indicates the proportion of each component by weight. In this case, polymer 1 accounts for 20% of the weight, while polymer 2 accounts for 80% of the weight. The volume fraction, on the other hand, represents the proportion of each component by volume. Here, the volume fraction for polymer 1 is given as 0.5.
To find the ratio of the density of polymer 1 to polymer 2, we need to consider their weight percentages and volume fractions.
First, let's calculate the weight fraction of polymer 1 by dividing its weight percentage by 100:
Weight fraction of polymer 1 = Weight percentage of polymer 1 / 100 = 20 / 100 = 0.2
Similarly, the weight fraction of polymer 2 can be calculated as:
Weight fraction of polymer 2 = Weight percentage of polymer 2 / 100 = 80 / 100 = 0.8
Next, we can use the formula relating weight fraction, volume fraction, and density:
Weight fraction = Volume fraction * Density
Substituting the known values, we can write the equation for polymer 1:
0.2 = 0.5 * Density of polymer 1
And for polymer 2:
0.8 = 0.5 * Density of polymer 2
Simplifying the equations, we find:
Density of polymer 1 = 0.2 / 0.5 = 0.4
Density of polymer 2 = 0.8 / 0.5 = 1.6
Finally, we can calculate the ratio of the density of polymer 1 to polymer 2:
Ratio = Density of polymer 1 / Density of polymer 2 = 0.4 / 1.6 = 0.25
Therefore, the ratio of the density of polymer 1 to polymer 2 is 0.25.
Learn more about polymer
brainly.com/question/14600435
#SPJ11
which description best fits the definition of activated complex? select the correct answer below: an activated complex is a catalyst present in a different phase from the reactants. an activated complex is a catalyst present in the same phase as the reactants. an activated complex is a molecule or ion produced in one step of a reaction mechanism and consumed in another. an activated complex is an unstable combination of reactant species representing the highest energy state of a reaction system.
Answers
The description that fits best is: "an activated complex is an unstable combination of reactant species representing the highest energy state of a reaction system."
A brief-lived species called an activated complex, commonly referred to as a transition state, develops during a chemical process. It is a highly energetic intermediate state where the reactant molecules are momentarily linked in a manner distinct from that of the starting materials or the products.
The activated complex immediately disintegrates into the products or returns to the reactants due to its instability. The energy barrier that must be crossed in order for the reaction to take place is represented by the activation energy, which is needed to produce the activated complex.
The activated compound is not a catalyst and is not separated from the reactants by a distinct phase. It is merely a transient, highly energetic intermediate state that develops during a chemical process. It cannot be directly viewed, but its presence can be deduced from theoretical calculations or experimental findings.
To know more about the activated complex refer here :
https://brainly.com/question/4358233#
#SPJ11
16) According to the Activity Series chemistry reference table, which of these metals will react most readily with
1.0 M HCl to produce H2(g)?
1) Zn
2) Mg
3) Ca
4) K
Answers
Answer:
The correct answer is (1). Zn has the highest electronegativity of all the metals in its column. It has a strong attraction for electrons and low ionization potential, so if potassium chloride were used instead of hydrochloric acid, potassium would react most readily.
Explanation:
This question is asking which metal will react most readily. According to the activity series, alkali metals are more reactive than alkaline earth metals. Magnesium has the lowest outer electron configuration of all the metals in its row on the periodic table so it will have a strong attraction for electrons. The magnesium atom therefore requires less energy to reach an outer energy level so it has the lowest first ionization potential. Therefore, magnesium will react most readily with HCl.
The balanced equation shows how sodium chloride reacts with silver nitrate to form sodium nitrate and silver chloride.
NaCl + AgNO3 Right arrow. NaNO3 + AgCl
If 4.00 g of NaCl react with 10.00 g of AgNO3, what is the excess reactant?
AgCl
NaCl
AgNO3
NaNO3
Answers
The excess reactant in this reaction is AgNO3.
To determine the excess reactant, we need to compare the amount of each reactant to the stoichiometric ratio given by the balanced equation. The molar mass of NaCl is 58.44 g/mol, and the molar mass of AgNO3 is 169.87 g/mol. We can calculate the moles of NaCl and AgNO3 using their respective masses:
Moles of NaCl = 4.00 g / 58.44 g/mol = 0.0685 mol
Moles of AgNO3 = 10.00 g / 169.87 g/mol = 0.0589 mol
According to the balanced equation, the stoichiometric ratio between NaCl and AgNO3 is 1:1. This means that 0.0685 moles of NaCl should react with 0.0685 moles of AgNO3. However, we have 0.0589 moles of AgNO3, which is less than the required amount. Therefore, AgNO3 is the limiting reactant.
Since AgNO3 is the limiting reactant, it will be completely consumed in the reaction, and some NaCl will be left over. Hence, NaCl is the excess reactant in this reaction.
for such more questions on reaction
https://brainly.com/question/25769000
#SPJ8
1. Unfortunately, the NYC watershed program, which naturally filters out pollutants from
rain and other water sources, has been polluted beyond repair. It normally provides 1.2
billion gallons of drinking water to the area every day¹. In its place, people will have to
buy Brita water filters that cost $26.99 and are only good for 40 gallons2. What is the
cost to replace the NYC watershed program with Brita water filters?

Answers
The cost per day to the US for relacing the NYC Watershed program with the Brita Water Filters is 3.5424375 billion US$ / day
What is the computation for the above answer?We know the following:
1.2 billion gallons of drinking water is provided every day under the NYC Watershed programFor every 40 gallons processed, people must spend $26.99 gallons.To arrive at the cost of replacing the NYC program, we say
1,200,000,000 gallons/40 gallons
= 30,000,000 gallons
The total cost of personal filtration =
30,000,000 * 26.99
= $809,700,000
Or Eight Hundred and Nine Million, Seven Hundred thousand United States dollars.
Given that the average consumer of water per capita in the US is 175 gallons per day
We say, 175/40 = 4.375
Hence the daily spending for the program will be
$809,700,000 * 4.375
= 3.5424375 billion US$ / day
Learn more about the Watershed:
https://brainly.com/question/13313239
#SPJ1
which particle is not used in calculating the atomic mass?
Answers
The smallest and least massive atomic particle is the electron, which is negatively charged.Due to the electron's extremely small mass, it is not counted inside the element's atomic number.
What particle does not add to mass?Although they are very small and possess a mass of 1/1850 of that of a protons or neutron, electrons carry a negative charge.Since they are so tiny, they do not, in reality, add to the weight of the atom.
What three particles make up an atom?These particles are frequently referred to it as subatomic particles since they are the building blocks of atoms.There are protons, neutrons, and electrons, three types of subatomic particles.Protons and electrons, two of a subatomic particles, each have an electrical charge of one or the other.
To know more about electron visit:
https://brainly.com/question/1255220
#SPJ4
if you add 15 grams of sodium chloride to 250 grams of water, what will the freezing and boiling points of the resulting solution be? hint: use the molal boiling point elevation and freezing point depression constants chart to find the k values.
Answers
The solution's boiling point will be 101.5 C. Freezing point depression = 2 * 1.027 * kg * mol * kg * mol * = 3.82 K. Thus, the solution's fusion point will be 3.8 C.
Why does NaCl solution freeze at a lower temperature than water does?The vapour pressure drops when a non-volatile solute is dissolved in a solvent. A lower temperature is needed for the solvent to freeze as a result.
Why does freshwater freeze more quickly than seawater?Due to the salt in seawater, it freezes at a lower temperature than fresh water—approximately 28.4 degrees Fahrenheit. Yet, because only the water part of seawater freezes, very little salt is present in the ice when it is formed.
To know more about Freezing point visit:-
https://brainly.com/question/1550397
#SPJ1
at 100.∘c, the ion product of water is 5.13×10−13. what is the concentration of hydronium ions at this temperature?
Answers
The concentration of hydronium ions at 100°C is 7.16 x 10⁻⁷ mol/L.
The ion product of water at 100°C is 5.13 x 10⁻¹³.
We can use this information to calculate the concentration of hydronium ions at this temperature. The ion product of water, or Kw, is defined as the product of the concentration of hydrogen ions (H+) and hydroxide ions (OH-) in water. This relationship is expressed as follows:
Kw = [H+][OH-, ]At 100°C, Kw = 5.13 x 10⁻¹³. Since pure water is neutral, the concentration of hydrogen ions (H+) is equal to the concentration of hydroxide ions (OH-).
Therefore, we can write:[H+] = [OH-]
To solve for [H+], we need to take the square root of Kw:[H+] = √(Kw)[H+] = √(5.13 x 10^-13)[H+] = 7.16 x 10⁻⁷ mol/L
Therefore, the concentration of hydronium ions at 100°C is 7.16 x 10⁻⁷ mol/L.
Learn more about hydronium ions at: https://brainly.com/question/1396185
#SPJ11
The dog has a mass of 57kg and the boy has a mass of 48 kg. Who has more kinetic energy?

Answers
Answer:
The Dog
Explanation:
The more mass something has the more kinetic energy it has in it.
Barium metal was quantitatively precipitated from a 1. 52 g sample of bacl2∙2h2o. The mass of the barium that was collected was 0. 844 g. Calculate the experimental mass percent of barium in the sample.
Answers
The sample's experimental mass percent of barium is roughly 55.526%.
Divide the mass of the barium collected by the beginning mass of the sample, then multiply the result by 100 to determine the experimental mass percent of barium in the sample.
The sample's initial mass (bacl22h2o) was 1.52 g.
Barium obtained weighs 0.844 g.
We must first calculate the mass of barium present in the combination bacl22h2o. This can be accomplished by deducting the sample's original mass from the mass of the water molecule (2H2O).
To determine the water's mass:
H2O's molecular weight is equal to 2(1.00794 g/mol) plus 16.00 g/mol, or 18.01528 g/mol.
36.03056 g/mol 2H2O = 2(18.01528 g/mol)
Mass of bacl2 equals initial sample mass minus mass of 2H2O
(Rounded to three decimal places) Mass of bacl2 = 1.52 g - 36.03056 g/mol = 1.52 g - 36.031 g = -34.511 g
The experimental mass percent of barium is then determined:
(Mass of barium collected / Initial mass of the sample) x 100 is the formula for the experimental mass percent of barium.
Experimental mass % of barium is equal to (0.844 g/1.52 g) multiplied by 100.
(Rounded to three decimal places) Experimental mass % of barium = 55.526 g
As a result, this figure shows the proportion of barium in the sample's mass.
To learn more about barium click here:
https://brainly.com/question/27233560#
#SPJ11
A toxin that binds specifically to voltage-gated sodium channels in axons would be expected to.
Answers
Hi, I hope this helped :)
compared to bromination, the of an alkane is not particularly selective. alkyl radical formation is for chlorination, which causes the transition state to be less dependent on the stability of the free radical that results from hydrogen abstraction. multiple choice question.
Answers
The bromination of an alkane, compared to the radical formation for chlorination takes place at a slower rate.
Alkanes are organic group of compounds that contains an aldehyde in their first carbon atom. Bromination and chlorination are the replacement of hydrogen group with a halogen group, such as bromine or chlorine.
As we know that halogenation involves the formation of a carbon-halogen bond. The halogenation will surely depend on the carbon-halogen strength. The more powerful the bond is the slower it will take place.
Bond formation energy of C−Cl is higher than that of C−Br bond. So, the ease of bromination is lower than the ease of chlorination. As chlorine is a more electro negative ion it gets dissociates easily.
Learn more about halogenation from,
https://brainly.com/question/14703081?referrer=searchResults
#SPJ4
Calculate the enthalpy of solution per mole NaOH (s) to form a 1.0 M NaOH solution. The mass of the solution is about 52 g (H2O + NaOH). The specific heat of the NaOH solution is about 3.90 J g-1 °C-1. Show all of your work.
Answers
The enthalpy of solution per mole NaOH (s) is 0.075 J.
Enthalpy, belonging to a thermodynamic gadget, is the sum of the machine's inner strength and the made of its stress and volume. it's far a state function used in many measurements in chemical, organic, and bodily systems at a steady pressure, which is without problems furnished by way of the massive ambient environment.
Calculation:-
H = mΔHf
ΔHf = H/m
= 3.90/52
= 0.075
Enthalpy is the quantity of inner electricity contained in a compound while entropy is the amount of intrinsic disorder inside the compound. Enthalpy is zero for elemental compounds consisting of hydrogen gas and oxygen gas; consequently, enthalpy is nonzero for water (no matter segment.
In thermochemistry, the enthalpy of solution is the enthalpy exchange related to the dissolution of a substance in a solvent at regular strain resulting in limitless dilution. The enthalpy of the answer is most usually expressed in kJ/mol at a steady temperature.
Learn more about enthalpy here:-https://brainly.com/question/14291557
#SPJ9
(a) Explain why ethylenediaminetetraacetic acid (EDTA) is the most widely used chelating agent in titrations. (2 marks) (b) The concentration of a solution of EDTA was determined by standardizing against a solution of Ca²+ prepared using a primary standard of CaCO3. A 0.3571 g sample of CaCO3 was transferred to a 500 mL volumetric flask, dissolved using a minimum of 6 M HCI, and diluted to 500 mL volume. After transferring a 50.00 mL portion of this solution to a 250 mL conical flask, the pH was adjusted by adding 5 mL of a pH 10 NH3- NH4Cl buffer containing a small amount of Mg-EDTA. After adding calmagite as an indicator, the solution was titrated with the EDTA and 42.63 mL was required to reach the end point. Calculate the molar concentration of EDTA in the titrant. (8 marks)
Answers
(a) EDTA is the most widely used chelating agent in titrations due to its ability to form stable complexes with a wide range of metal ions, including those of calcium, magnesium, iron, and zinc. (b) the molar concentration of the EDTA titrant is 0.008391 M.
a) The stability constants of these complexes are high, which means that EDTA can effectively chelate metal ions even in dilute solutions. Additionally, EDTA has a relatively low molecular weight and can be easily dissolved in water, making it a convenient and versatile chelating agent for titrations.
(b) First, we need to calculate the molar concentration of Ca²+ in the solution. The mass of CaCO3 used to prepare the solution is:
mass of CaCO3 = 0.3571 g
The molar mass of CaCO3 is:
molar mass of CaCO3 = 100.09 g/mol
Using these values, we can calculate the number of moles of CaCO3:
moles of CaCO3 = mass of CaCO3 / molar mass of CaCO3
= 0.3571 g / 100.09 g/mol
= 0.003569 mol
Since the solution was diluted to a final volume of 500 mL, the molar concentration of Ca²+ is:
molar concentration of Ca²+ = moles of CaCO3 / final volume
= 0.003569 mol / 0.500 L
= 0.007138 M
During the titration, the EDTA reacts with the Ca²+ ions in the solution according to the following stoichiometry:
Ca²+ + EDTA⁴⁻ → CaEDTA²⁻
To determine the molar concentration of EDTA, we need to use the volume of EDTA solution required to reach the end point of the titration. This volume is:
volume of EDTA solution = 42.63 mL = 0.04263 L
We also know that the molar concentration of Ca²+ in the solution is 0.007138 M. Since the stoichiometry of the reaction is 1:1, the moles of EDTA used in the titration are equal to the moles of Ca²+ in the solution. Therefore, the molar concentration of EDTA is:
molar concentration of EDTA = moles of EDTA / volume of EDTA solution
= moles of Ca²+ / volume of EDTA solution
= molar concentration of Ca²+ × volume of Ca²+ solution / volume of EDTA solution
= 0.007138 M × 0.05000 L / 0.04263 L
= 0.008391
learn more about molar mass here:
https://brainly.com/question/22997914
#SPJ11
In the previous step, you determined
0.25 mol HCI reacts. The molar mass
of Mg is 24.31 g/mol.
What mass of Mg is required?
PLEASE HELP ASAP

Answers
Approximately 3.04 grams of magnesium would be required to react with 0.25 moles of hydrochloric acid.
To determine the mass of Mg required, we need to use the balanced chemical equation for the reaction between hydrochloric acid (HCl) and magnesium (Mg):
2HCl + Mg → MgCl2 + H2
From the balanced equation, we can see that 2 moles of HCl react with 1 mole of Mg. Therefore, if 0.25 mol of HCl reacts, we would need half of that amount, which is 0.125 mol of Mg.
To calculate the mass of Mg required, we need to multiply the number of moles of Mg by its molar mass. The molar mass of Mg is given as 24.31 g/mol. Therefore, the mass of Mg required can be calculated as follows:
Mass of Mg = Number of moles of Mg × Molar mass of Mg
Mass of Mg = 0.125 mol × 24.31 g/mol
Mass of Mg = 3.04 g
For such more questions on moles
https://brainly.com/question/19964502
#SPJ8
HELP PLS ITS URGENT what is the energy of a photon of a red light?
red: 6.50x10^-7m
Answers
Answer:
I think the answer is is 4.62x10^14 s-1
Explanation:
Sorry if it is wrong
in this lesson, we’ve mostly focused on oxygen and nitrogen partial pressures. what else (other than o2 and n2) may contribute to the total pressure in the alveoli?
Answers
In addition to oxygen (O2) and nitrogen (N2), other gases that contribute to the total pressure include carbon dioxide (CO2), water vapor (H2O), and traces of other gases such as argon (Ar), helium (He), and neon (Ne).
Carbon dioxide is produced as a waste product of cellular metabolism and is transported from the tissues to the lungs for exhalation. It contributes to the partial pressure of gases in the alveoli.
Water vapor is produced through the process of respiration and adds to the total pressure. As we breathe, the air gets humidified in the respiratory tract, leading to the presence of water vapor in the alveoli.
Traces of other gases, such as argon, helium, and neon, are present in the atmosphere in small quantities and can also contribute to the total pressure in the alveoli. However, their concentrations are much lower compared to oxygen and nitrogen.
To learn more about water
https://brainly.com/question/30982742
#SPJ11
How many joules are required to change 225 g of ice at 0 °C to steam at 100 °C?
Answers
Answer:The answer is
153.7
k
J
.
What you are asked to determine is the total energy required to go from ice to water, and then from water to vapor - the phase changes underwent by the water molecules.
In order to do this, you'll need to know:
Heat of fusion of water:
Δ
H
f
=
334
J
/
g
;
Heat of fusion vaporization of water:
Δ
H
v
=
2257
J
/
g
;
Specific heat of ice:
c
=
2.09
J
/
g
∘
C
;
Specific heat of water:
c
=
4.18
J
/
g
∘
C
;
Specific heat of steam:
c
=
2.09
J
/
g
∘
C
;
So, the following steps describe the overall process:
1. Determine the heat required to raise the temperature of the ice from
−
15.0
∘
C
to
0
∘
C
:
q
1
=
m
⋅
c
i
c
e
⋅
Δ
T
=
50.0
g
⋅
2.09
J
g
⋅
∘
C
⋅
(
0
∘
C
−
(
−
15
∘
C
)
)
=
1567.5
J
2. Determine the heat required to convert
0
∘
C
ice to
0
∘
C
water:
q
2
=
m
⋅
Δ
H
f
=
50.0
g
⋅
334
J
g
=
16700
J
3. Determine the heat required to go from water at
0
∘
C
to water at
100
∘
C
:
q
3
=
m
⋅
c
w
a
t
e
r
⋅
Δ
T
=
50.0
g
⋅
4.18
J
g
⋅
∘
C
⋅
(
100
∘
C
−
0
∘
C
)
=
20900
J
4. Determine the heat required to convert
100
∘
C
water to
100
∘
C
vapor:
q
4
=
m
⋅
Δ
H
v
=
50.0
g
⋅
2257
J
g
=
112850
J
5. Determine the heat required to go from
100
∘
C
vapor to
120
∘
C
vapor:
q
5
=
m
⋅
c
v
a
p
o
r
⋅
Δ
T
=
50.0
g
⋅
2.09
J
g
⋅
∘
C
⋅
(
120
∘
C
−
100
∘
C
)
=
2090
J
Therefore, the total heat required is
q
T
O
T
A
L
=
q
1
+
q
2
+
q
3
+
q
4
+
q
5
=
152696.5
J
=
153.7
k
J
Explanation:
the reaction of carbon with oxygen to produce carbon monoxide is an example of which class of reaction?
Answers
It is combination reaction.
What is combination reaction?
Two reactants undergo chemical reactions to produce a single product in combination reactions. Combination reactions are prevalent in many natural oxidation processes.
Complete solution, step by step:
Two reactants combine to create one product in a reaction known as a combination reaction.
A and B are the reactants, and C is the end result of the chemical reaction, therefore A+BC.
Combination reactions are prevalent in many natural oxidation processes. Likewise, examples of combination reactions are those in which the reactants include metals and halogens. Halogens or oxygen can provide suitable anions, while metals can create suitable cations. As a result, it is also frequently seen that combination reactions are ionic reactions. The rust we observe on iron objects is primarily an oxidation reaction as well as the previously described combination reaction.
It goes like this:
4Fe+3O22Fe2O3.
Rust, which is nothing more than ferrous oxide, is created by a balanced chemical reaction. Another reaction is the H2+Cl22HCl reaction between the highly reactive halogen chlorine and hydrogen. Hydrogen chlorine, a common acid in many chemical reactions, is the only result of this reaction.
These reactions can occur between various metals and reactive non-metals, such halogens, for example. Other instances of covalent bonding include the synthesis of sulphur dioxide from the reaction of sulphur and oxygen.
Learn more about combination reaction from given link
https://brainly.com/question/15092182
#SPJ4
Determine the volume (in mL) of water that needs to be added to 25.0 mL of a 0.250 M NaBr to produce a 0.0435 M solution. Assume the volumes are additive.
Answers
The volume of water added to make the diluted solution is 118.68 mL.
To solve this question, we'll begin by calculating the volume of the diluted solution. This can be obtained as follow:
Volume of stock solution (V₁) = 25 mL
Molarity of stock solution (M₁) = 0.250 M
Molarity of diluted solution (M₂) = 0.0435 M
Volume of diluted solution (V₂) =?The volume of the diluted solution can be obtained as follow:
M₁V₁ = M₂V₂0.25 × 25 = 0.0435 × V₂
6.25 = 0.0435 × V₂
Divide both side by 0.0435V₂ = 6.25 / 0.0435
V₂ = 143.68 mL
Thus, the volume of the diluted solution is 143.68 mL
Finally, we shall determine the volume of water added to make the diluted solution.
Volume of stock solution (V₁) = 25 mL
Volume of diluted solution (V₂) = 143.68 mL
Volume of water added =?
Volume of water added = (Volume of diluted solution) – (Volume of stock solution)Volume of water added = 143.68 – 25
Volume of water added = 118.68 mLTherefore, the volume of water added to make the diluted solution is 118.68 mL
Learn more: https://brainly.com/question/24869996
In this periodic table, which type of element is shown in green boxes?
A. Noble gases
B. Metalloids
C. Nonmetals
D. Metals

Answers
The correct answer is B. Metalloids
Explanation:
In this periodic table, the green boxes include elements such as Boron (B), Arsenic (As), and Antimony (Sb). All these elements are known in chemistry as semimetals or metalloids. This classification is because these elements cannot be completely classified into metals or non-metals. Moreover, they all display similar properties including some level of conductivity, malleability, and solid-state. According to this, the correct answer is B.
Answer:
B. Metalloids
Explanation:
What is the correct equilibrium constant expression for the following reaction?
2NCl3(g) Û N2g) + 3Cl2g
Answers
Answer:
K = [N2] [Cl2]³ / [NCl3]²
Explanation:
The equilibrium expression, K, of a reaction:
aA + bB ⇄ cC + dD
Is defined as the ratio between the multiplication of concentrations of products powered to its reaction coefficient and the multiplication of concentrations of reactants powered to its reaction coefficient as follows:
K = [C]^c[D]^d / [A]^a[B]^b
Now, for the reaciton:
2NCl3 ⇄ N2(g) + 3Cl2(g)
K is:
K = [N2] [Cl2]³ / [NCl3]²
A child sits on a partially filled balloon and decreases the volume by half. What happens to the pressure inside of the balloon?
It increases
It decreases
It stays the same
Answers
**Check for proof photos at the bottom.**
**Answers are in bold.**
__________________________________________________________
A child sits on a partially filled balloon and decreases the volume by half. What happens to the pressure inside of the balloon?
A. It increases
For the same balloon, how does the density inside the balloon change?
A. increases
A scientist takes a small, partially inflated balloon out of liquid nitrogen (at a very low temperature). As the balloon rests on the table, it begins to grow in size. Which statement(s) best explains why the balloon grows?
B. The air warms and increases particle movement. Increased particle movement leads to increased volume.
C. Increased temperature means increased volume as long as the container is flexible.
__________________________________________________________
Answer the following questions with or without using a calculator.
A balloon contains 1.00 mol helium gas at STP. What will the volume of the balloon be if the pressure increases to 2.00 atm?
✔ 11.2 L
A syringe contains 0.10 mol neon gas at STP. What volume does the syringe indicate?
✔ 2.24 L
A chemist adds 0.10 mol argon gas to the syringe. The pressure and temperature remain constant. What will be the volume on the syringe after the argon is added?
✔ 4.48 L
__________________________________________________________
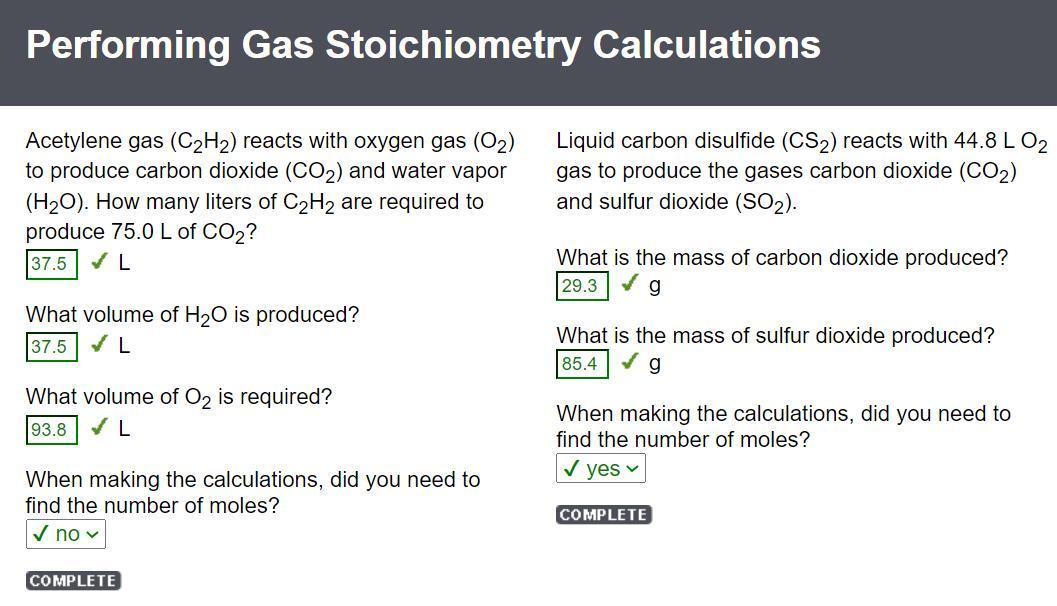
Acetylene gas (C2H2) reacts with oxygen gas (O2) to produce carbon dioxide (CO2) and water vapor (H2O). How many liters of C2H2 are required to produce 75.0 L of CO2?
37.5 L
What volume of H2O is produced?
37.5 L
What volume of O2 is required?
93.8 L
When making the calculations, did you need to find the number of moles?
✔ no
Liquid carbon disulfide (CS2) reacts with 44.8 L O2 gas to produce the gases carbon dioxide (CO2) and sulfur dioxide (SO2).
What is the mass of carbon dioxide produced?
29.3 g
What is the mass of sulfur dioxide produced?
85.4 g
When making the calculations, did you need to find the number of moles?
✔ yes
__________________________________________________________
Explanation:The child is putting pressure on the balloon, and squishing particles together when he/she sits on it, thus increasing pressure. Since particles are being squished closer together, density also increases.
Inflated objects will grow in size when the air inside is hotter. This is because particles move more rapidly. An example would be bike tires being more inflated under hot conditions.
One mole of any gas at standard temperature and pressure (STP) will occupy 22.4 L. If pressure increases, the volume decreases, and vice versa. If moles decreases, so will the volume, and vice versa.
Here are photos of Edge just incase.


The pressure inside of the balloon will Increase. Hence The correct option is (A).
What is Boyle's Law ?
According to this law, the pressure (p) of a given quantity of gas varies inversely with its volume (v) at constant temperature; i.e., in equation form,
pv = k
Where K = constant.
Initial pressure = P₁Initial volume = V₁Final pressure =P₂Final volume = V₂If we consider that temperature of the gas which filled inside the balloon is constant.
We know that , at constant temperature
P₁V₁ = P₂V₂
Given that
V₂ = V₁ / 2
P₂ = P₁V₁/V₂
P₂ = P₁V₁/V₁/2
P₂ = 2P₁
Therefore, the pressure inside the balloon will become double.Hence The correct option is (A).
Learn more about Gas Law here ;
https://brainly.com/question/12669509
#SPJ2
One problem with fuel-cell cars is that
gas is not readily available in
pure form.
Answer here

Answers
Answer: Hydrogen.
Explanation: One problem with fuel-cell cars is that hydrogen gas is not readily available in pure form. Fuel cell vehicles use hydrogen gas to power an electric motor. Unlike conventional vehicles which run on gasoline or diesel, fuel cell cars and trucks combine hydrogen and oxygen to produce electricity, which runs a motor. Hope this helps ^-^.
Answer:
Hydrogen.
Explanation:
Electrolysis is performed upon molten MgCl2. platinum electrodes are used. (a) write the cathode and anode half reactions

Answers
Electrolysis occur when an electric current is passed through a liquid or solution thereby causing the decomposition of chemicals.
For magnesium chloride, it will be heated to be able to conduct electricity. When molten, the MgCl2 will decomposes into Mg2+ and 2Cl- ions. During electrolysis, the elements are separated according to the equations;
\(\begin{gathered} Mg^{2+}+2e^-\rightarrow Mg(s) \\ 2Cl^-\rightarrow Cl_2(g) \end{gathered}\)According to the half reactions, the Mg2+ is reduced at the cathode (-) and the Cl- is oxidized at the anode (+). Reduction at the cathode shows that magnesium gains two electrons to form a magnesium solid while the chlorine ion looses two electrons at the anode
ANYONE HELP ME IN CHEMISTRY:(
JUST THE NAME AND THE SYMBOL ANYONE WHO CAN HELP ME:(

Answers
Answer:
Answers are as follows
Explanation:
Hydrogen: H
Scandium: Sc
Carbon: C
Nitrogen: N
Ti: Titanium
Pt: Platinum
Be: Beryllium
Li: Lithium
F: Fluorine
Hg: Mercury
Pb: Lead
Vanadium: V
Oxygen: O
Boron: B
Arsenic: As
Selenium: Se
Uranium: U
Gold: Au
What is the ph of a solution of h2so4 that has [h3o ] = 5.45 × 10–5 m? round to the nearest hundredth.
Answers
The pH of a solution of H₂SO₄ that has [H₃O⁺] is 5.45 × 10⁻⁵ M, is 4.26.
What is pH?pH is define as the negative logarithm of the concentration of H⁺ or H₃O⁺ ions, and it is represented as:
pH = -log[H⁺]
Concentration of H₃O⁺ in H₂SO₄ solution = 5.45 × 10⁻⁵ M
pH of H₂SO₄ solution will be calculated as:
pH = -log(5.45 × 10⁻⁵)
pH = -(-4.26)
pH = 4.26
Hence required pH is 4.26.
To know more about pH, visit the below link:
https://brainly.com/question/522383
Answer:
4.26 is the answer
Explanation:
edge 2022
Help please? I really need to finish this. A response would be helpful :)

Answers
Explanation: Tissues are groups of similar cells that have a common function
Answer:
do you have options that came with it
Explanation:
Which equation relates ΔG, Keq, and ln
Answers
The equation that relates ΔG, Keq, and ln is ΔG = -RT ln(Keq), where ΔG is the change in Gibbs free energy, Keq is the equilibrium constant, R is the gas constant, and T is the temperature.
The relationship between Gibbs free energy, equilibrium constant, and natural logarithm can be described by the equation ΔG = -RT ln(Keq), which is also known as the Gibbs-Helmholtz equation. This equation is used to calculate the change in Gibbs free energy during a chemical reaction, based on the equilibrium constant and temperature.
The negative sign in front of RT ln(Keq) indicates that a negative change in Gibbs free energy (ΔG) corresponds to a favorable reaction, while a positive ΔG indicates an unfavorable reaction. The equation can also be rearranged to solve for Keq or temperature, depending on the variables given in the problem.
For more questions like Reaction click the link below:
https://brainly.com/question/28984750
#SPJ11
Gas exchange, which is necessary for photosynthesis, can occur most easily in which leaf tissue?
Answers
Gas exchange, which is necessary for photosynthesis, can occur most easily in the spongy mesophyll leaf tissue.
The mesophyll tissue is composed of the palisade mesophyll and spongy mesophyll layers, which are responsible for photosynthesis. The palisade mesophyll is located on the upper surface of the leaf and contains tightly packed chloroplasts, while the spongy mesophyll is located below the palisade mesophyll and has a more loosely arranged structure that allows for better gas exchange.
The exchange of gases required for photosynthesis, such as carbon dioxide and oxygen, occurs through small pores on the leaf surface called stomata. These stomata are more concentrated on the underside of the leaf, particularly in the spongy mesophyll layer, allowing for efficient gas exchange.
To learn more about photosynthesis, click here:
https://brainly.com/question/29764662
#SPJ11