Answers
(A) By far the most common isotope of carbon is carbon- 12( 12C), which contains six neutrons in addition to its six protons.
Also there are more Carbon- 12 in atmosphere than Carbon-13 because matter is amended in carbon- 12, because its lighter weight is used in stormy emigrations are amended in carbon- 13. The rate of carbon- 13 to carbon- 12 in the atmosphere and the ocean are roughly the same.
(B) Boron- 11 is more abundant. Boron is linked as titles containing five protons in the nexus. This means that boron- 10 would have five neutrons in the nexus and boron- 11 would have six protons.
(C) Given that isotope of Bromine with the at. mass, 79u has 49.7 composition. And also the isotope of Bromine with the at. mass, 81u hasv50.3 composition.
Since, donation of Bromine- 79 towards the at. mass is
Mass = ( 79 *49.7)/ 100 = 39.263
donation of Bromine- 81 towards the at. mass is
Mass = ( 81 *50.3)/ 100 = 40.743
Average at. mass = 39.2640.74 = 80.0068 amu
(D) The average atomic mass of an element is the sum of the millions of its isotopes, each multiplied by its natural cornucopia( the numeric associated with percent of tittles of that element that are of a given isotope).
To know more about atomic mass here :
https://brainly.com/question/17067547?referrer=searchResults
#SPJ1
Related Questions
The graph shows the changes in the phase of ice when it is heated which of the following temperatures describes the value of A

Answers
Answer:
Less than 0 °C, because B represents the temperature at which ice melts.
Explanation:
Answer: b. 100 °C, because it is the boiling point of water.
Explanation: i got it right on the test
5. given the ratio of reactants, what is the possibility of obtaining di- and polychlorinated product? explain.
Answers
The ratio of reactants is chlorination of 2,3 dimethyl butane the possibility of obtaining do and the polychlorinated product is not seen.
When a mixture of methane and chlorine is exposed to ultraviolet light a substitution reaction occurs and the organic product is chloromethane. Because there are various hydrogen atoms that can be extracted in the first propagation step.
Abstraction of a hydrogen atom from the middle carbon of propane results in 2-chloropropane. In the presence of sunlight, methane reacts with chlorine to form chloromethane. The chlorination of methane is a free radical substitution reaction. Chlorine cannot turn into free radicals in the dark, so no reaction takes place. Therefore, the presence of sunlight is essential for the reaction to proceed.
Learn more about The reactants here:- https://brainly.com/question/6421464
#SPJ4
do resonance structures always contribute equally to the overall structure of a molecule?
Answers
No, resonance structures do not always contribute equally to the overall structure of a molecule. Some resonance structures may be more stable than others, meaning they contribute more to the actual structure of the molecule.
Resonance structures are another way of representing electron distribution in molecules. They are used to describe the delocalization of electrons within a molecule or ion. At resonance, electrons are spread across multiple atoms or bonds and are not localized to a single bond.
However, it is important to note that resonance structures do not represent the actual physical state of the molecule. These are just different ways to draw Lewis structures of molecules or ions to show the distribution of electrons.
Furthermore, resonance structures do not necessarily contribute equally to the overall structure of a molecule. In some cases, one resonance structure may be more stable or important than another, contributing more to the actual electronic structure of the molecule.
To learn more about resonance structures:
https://brainly.com/question/6780213
https://brainly.com/question/15060424
PLEASE HELP HELP ME. THIS IS DUE TODAY PLEASE

Answers
Answer:
B, C
Explanation:
Hope it helps i read it all
What is the condensed formula for 2-pentyne??
Answers
Answer:
C5H8
Explanation:
The condensed formula is also known as structural formula of a chemical compound which shows a graphical representation of the molecular structure that represents, the arrangement of the atoms in the three-dimensional space.
The condensed formula of 2-pentyne is C5H8.
There is a triple bond between two carbon atoms at second carbon as shown in the figure.

All the following are true EXCEPT
A. Atomic radius of Na < atomic radius of Mg
B. electronegativity of C > electronegativity of B
C. 1st ionization energy of K > first ionization energy of Rb.
D. lonic radius of Br"> atomic radius of Br
Answers
Answer:
D) ionic radius of Br">atomic radius of br
CH3COOH CH3COO– + H+
You start with 0.05 moles of acetic acid in 500 mL of water. At equilibrium, the pH of the solution is 2.873. What is the equilibrium constant of this reaction? Hint: You will need to calculate an antilog using a scientific calculator.
Answers
(a)
pH = 4.77
; (b)
[
H
3
O
+
]
=
1.00
×
10
-4
l
mol/dm
3
; (c)
[
A
-
]
=
0.16 mol⋅dm
-3
Explanation:
(a) pH of aspirin solution
Let's write the chemical equation as
m
m
m
m
m
m
m
m
l
HA
m
+
m
H
2
O
⇌
H
3
O
+
m
+
m
l
A
-
I/mol⋅dm
-3
:
m
m
0.05
m
m
m
m
m
m
m
m
l
0
m
m
m
m
m
l
l
0
C/mol⋅dm
-3
:
m
m
l
-
x
m
m
m
m
m
m
m
m
+
x
m
l
m
m
m
l
+
x
E/mol⋅dm
-3
:
m
0.05 -
l
x
m
m
m
m
m
m
m
l
x
m
m
x
m
m
m
x
K
a
=
[
H
3
O
+
]
[
A
-
]
[
HA
]
=
x
2
0.05 -
l
x
=
3.27
×
10
-4
Check for negligibility
0.05
3.27
×
10
-4
=
153
<
400
∴
x
is not less than 5 % of the initial concentration of
[
HA
]
.
We cannot ignore it in comparison with 0.05, so we must solve a quadratic.
Then
x
2
0.05
−
x
=
3.27
×
10
-4
x
2
=
3.27
×
10
-4
(
0.05
−
x
)
=
1.635
×
10
-5
−
3.27
×
10
-4
x
x
2
+
3.27
×
10
-4
x
−
1.635
×
10
-5
=
0
x
=
1.68
×
10
-5
[
H
3
O
+
]
=
x
l
mol/L
=
1.68
×
10
-5
l
mol/L
pH
=
-log
[
H
3
O
+
]
=
-log
(
1.68
×
10
-5
)
=
4.77
(b)
[
H
3
O
+
]
at pH 4
[
H
3
O
+
]
=
10
-pH
l
mol/L
=
1.00
×
10
-4
l
mol/L
(c) Concentration of
A
-
in the buffer
We can now use the Henderson-Hasselbalch equation to calculate the
[
A
-
]
.
pH
=
p
K
a
+
log
(
[
A
-
]
[
HA
]
)
4.00
=
−
log
(
3.27
×
10
-4
)
+
log
(
[
A
-
]
0.05
)
=
3.49
+
log
(
[
A
-
]
0.05
)
log
(
[
A
-
]
0.05
)
=
4.00 - 3.49
=
0.51
[
A
-
]
0.05
=
10
0.51
=
3.24
[
A
-
]
=
0.05
×
3.24
=
0.16
The concentration of
A
-
in the buffer is 0.16 mol/L.
hope this helps :)
The equilibrium constant of this reaction is 1.80×10-5
Given data,
pH of solution = 2.873
Number of moles of acetic acid (m) = 0.05 moles
Volume of water (V) = 500 mL = 0.5L
So, concentration (C) = m/V in lit = 0.05/0.5 = 0.1 M
Equilibrium constant ( K ) = \([CH_{3} COO-]\)×\([H+_{} ]\)/\([CH_{3} COOH]\)
Since, acetic acid is weak acid,
So, Equilibrium constant ( K ) = \([H+]^{2}\)/\([CH_{3} COOH]\) ....(i)
As the pH = 2.873, the \([H+_{} ]\) is antilog of -2.873 or 1.34×10-3 M.
Putting the value of concentration of \(H+_{}\) and \(acetic_{} acid\) in equation (i).
Equilibrium constant ( K ) = 1.80×10-5
What is weak acid ?The acid which is partially dissociates into ions on dissolving in aqueous solution is called weak acid.
Example: \(acetic_{} acid\).
To learn more about weak acid here.
https://brainly.com/question/12811944
#SPJ3
Help please !!!!!!!!!!!!!!!!

Answers
9.answer is 0¯²
10.answer is I¯
11. answer is AI+³
12.answer is Cs+
13.answer is Fe+²
14.answer isCI¯
Pleaseeee help I’m desperate
If u can answer all three of them
I’ll give you brainliest

Answers
Answer:
5.80
Explanation:
Benzene can be nitrated with a mixture of nitric and sulfuric acids. Draw the 3-atom electrophile in the reaction. Include any formal charges.
Answers
When benzene is nitrated with a mixture of nitric and sulfuric acids, the electrophile that attacks the benzene ring is a nitronium ion, which has the chemical formula NO2+. This electrophile is generated in situ from the reaction between nitric acid and sulfuric acid, as shown below:
HNO3 + H2SO4 → NO2+ + HSO4- + H2O
The nitronium ion has a formal positive charge on the nitrogen atom (+1) and a formal negative charge on one of the oxygen atoms (-1), giving it an overall formal charge of 0. The three atoms that make up the nitronium ion are nitrogen (N), oxygen (O), and oxygen (O), arranged in a linear configuration. The nitrogen atom is the electrophilic center, as it is the site of positive charge and the atom that attacks the benzene ring in the nitration reaction.
To know more about nitration reaction:
https://brainly.com/question/1626142
#SPJ11
Ill in the blank with the correct number to balance the equation: ___KClO3 → 2KCl 3O2. (Enter only a whole number. )
Numerical Anwer Expected!
Anwer for Blank 1:
Answers
Correct number to balance the equation: ___KClO3 → 2KCl 3O2 is "2".
We obtain KCl and oxygen gas from the breakdown of potassium chlorate (KClO3).
1.5 moles of O2 gas and one mole of KCl are produced from one mole of KClO3.
Therefore, the response will be:
KCL)3 - - > KCL + 3/2 O2
The entire equation will be multiplied by two in order to transform the fractional coefficient to a whole number.
2X [ KCLO3 - - > KCL + 3/2 O2 ]
A balanced equation will therefore be:
2KCLO3 - - > 2KCL + 3 O2
KClO3's coefficient is therefore "2".
To learn more about Equation Please click on the given link:
https://brainly.com/question/11904811
#SPJ4
SOMEONE HELP ILL BRAINLISTTTTTTY. Write in your own wordsss. I’ll give you 70 points and a brainlist in total. Which is enough write it in full sentence summarise a paragraph or so not too long but yeah make it make sense.
1) Give a specific example of a species of plant or animal that had been identified as ‘vulnerable’ or ‘endangered’ or ‘extinct’ as a result of water pollution. Give details - where, when and why did it happen?
Answers
Answer:
Explanation:
Hawksbill turtles were identified as endangered due to water pollution. Chemical pollutants can weaken turtle's immune systems making them prone to diseases. Plastic packaging, nylon fishing lines etc can be eaten by turtles or they may get stuck in them resulting in death.
sorry this is the only details i can give
HELP!! PLEASE 10+ points!
Can you tell from the masses of the reactants which one of the reactants will be the limiting reactant? Defend your answer (use an equation within your defense).
Answers
Answer:
Explanation:
so, limiting reactants are the reactant which limit the continuing of the reaction or in simple word they are the element that run out first and are totally consumed
equation :
2koh+h2so4⇒ k2so4 +2h2o
if we have 4 mol of h2so4 and 3 mol of 2koh
number of produced moles of k2so4 on consuming all :
h2so4 ⇒ k2so4
1 mol 1 mol
4 mol ?mol
no of mol of k2so4 if all h2so4 is consumed 4 mol
2koh⇒ k2so4
2 mol 1 mol
3 mol ?mol
no of mol of k2so4 if we consume all koh = 1.5 mol
since the koh produces less mol on consuming it all
the koh is the limiting reactant
What is the Hsol for KF →K+ + F-? The lattice energy is –784 kJ/mol, the enthalpy of hydration for Ki is –336
kJ/mol, and the enthalpy of hydration for F-is-431 kJ/mol. Use AHsol= - Hiat + Hydr
0-879 kJ/mol
0 -17 kJ/mol
O 17 kJ/mol
O 1,551 kJ/mol
Answers
Answer:
17 kJ/mol
Explanation:
got it on edge
Why does fumaric acid have a higher boiling point than maleic acid, even though they both can form hydrogen bonds?
Answers
Answer:.
Explanation:
Observations CuSO4 & NH4Cl Conventional, total ionic, net ionic
Answers
Therefore, the net ionic equation for the reaction is Copper(2+) (aq) + 2 chlorine- (aq) → Copper(II) chloride (aq).
What takes place when Copper(II) sulfate and Ammonium hydroxide interact?Ammonium sulphate and Copper hydroxide precipitate are the first products of the reaction between copper sulphate and ammonium hydroxide.
Mixing copper(II) sulphate and ammonium chloride results in the following observations:
Conventional: When copper ions (Copper2+) from Copper(II) sulfate are present, a blue solution develops. The colour of Ammonium Chloride doesn't seem to have changed at all.
Ionic total: While Ammonium Chloride dissociates into Ammonium and Chlorine- ions in solution, Copper(II) sulfate dissociates into Copper2+ and Sulfate 2- ions.
Copper(II) sulfate (aq) + 2 Ammonium Chloride (aq) → Copper(II) Chloride (aq) + 2 Ammonium (aq) + Sulfate 2- (aq)
Net Ionic: The net ionic equation shows only the species involved in the reaction. In this case, the Copper2+ and the Cl- ions combine to form Copper(II) chloride.
Copper2+ (aq) + 2 Chlorine- (aq) → Copper(II) chloride (aq)
To know more about ionic equation visit:-
https://brainly.com/question/29299745
#SPJ1
the solubility of sucrose at 70.0 oc is 320 g/100 g h2o. how much sucrose need to dissolve in 200 g of water at 70.0 oc to prepare a saturated solution?
Answers
Dissolving 640 g of sucrose in 20g of water (h2O) at 70°C will give us a saturated solution.
We know that at t degree Celcius the solubility of a solute in a solvent
=( weight of solute(g)/ weight of solvent(g)) * 100
Given at 70° C solubility of sucrose in h2o is 320g/100 g of h2o
Let x be the amount of sucrose that must dissolve in 200g of water at 70°C to give a saturated solution.
∴x = 320 * 200/100
⇒x = 640 g
∴ 640 g of sucrose dissolved in 200 gm of h2O at 70°C will give us a saturated solution.
Know more about saturated solutions:
brainly.com/question/1851822
#SPJ4
A scientist is investigating which type of medicine would dissolve faster, a solid pill or a scoop of powder. Which hypothesis
should the scientist develop given prior knowledge about surface area?
A. Solid pills have more surface area and will take longer to dissolve.
B. Powders have less surface area and will dissolve faster.
C. Solid pills have less surface area and will not dissolve.
D. Powders have more surface area and will dissolve faster.
Answers
B.
because a solute with a smaller surface area will dissolve faster
Student Name:.....
Q1.
The drawing below shows Rebekah pulling a turnip out of the ground.
B
(a)
Which arrow, A, B, C or D, shows the direction of force of Rebekah's hand on the
turnip?
1
1 mark
Answers
Answer:
ummm I really want to help with this problem but I think that you forgot the photo of Rebekah Pullin the turnip
State two things that happen to wave when it passes from one material into another material
Answers
Answer:Radio waves move through the air at the same speed as visible light, Sound waves are mechanical waves all sound waves regardless of there spee or frequency.
Explanation: I got my answer from stemscopes.
A 21.8 g sample of CaSO4 is found to contain 6.42 g of Ca and 10.2 g of O. Find the mass of sulfur in a sample of CaSO4 with a mass of 92.6 g.
Answers
The mass of sulfur in a sample of CaSO₄ with a mass of 92.6 g is 21.38 g.
We know that CaSO₄ is composed of calcium, sulfur, and oxygen. Therefore, the mass of sulfur can be calculated by subtracting the masses of calcium and oxygen from the total mass of CaSO₄ given as 92.6 g.
To find the mass of sulfur, we need to subtract the mass of calcium and oxygen from the total mass of CaSO₄. Mass of Ca = 6.42 g; mass of O = 10.2 g.
Hence, mass of Sulfur = Total mass - Mass of Ca - Mass of O = 92.6 g - 6.42 g - 10.2 g = 75.98 g.
Therefore, the mass of sulfur in a sample of CaSO₄ with a mass of 92.6 g is 21.38 g.
Learn more about sulfur here:
https://brainly.com/question/10982712
#SPJ11
Volume of water displaced

Answers
Answer:
The volume of displaced fluid is equivalent to the volume of an object fully immersed in a fluid.
In a chemical reaction where an electron is exchanged from one reactant to another, the structure that loses an electron is?
Answers
Answer:
Oxidized
Explanation:
Oxidation and reduction are terms used to describe reactions in which electrons move from one atom or molecule to another atom or molecule. Oxidation occurs when electrons are lost, while reduction occurs when electrons are gained. (The charge is reduced). The two processes are linked, whenever one substance is oxidized another has to be reduced.
Which reaction occurs when you add NaOH to the buffer solution? (Ac = acetate) a. Ac- + H3O+ <--> HAc + H2O b. OH- + H3O+ <--> 2 H2O c. HAc + OH- <--> Ac- + H2O d. Ac- + OH- <--> AcOH e. HAc + H3O+ <--> H2 + H2O + Ac- f. HAc + Ac- <--> Ac- + HAc g. HAc + H3O+ <--> Ac- + H2O
Answers
The reaction that occurs when you add NaOH to a buffer solution depends on the specific components of the buffer. However, one possible reaction is \(HAc + OH^- < -- > Ac^- + H_2O\). The correct answer is option c.
When you add NaOH (sodium hydroxide) to a buffer solution containing the acetate ion (\(Ac^-\)) and acetic acid (HAc), the reaction that occurs is the neutralization of the acidic component, HAc, by the hydroxide ion (\(OH^-\)). This neutralization reaction results in the formation of the acetate ion (\(Ac^-\)) and water (H\(_2\)O). The reaction can be represented as follows:
\(HAc + OH^- < -- > Ac^- + H_2O\)
In this reaction, the hydroxide ion (\(OH^-\)) from NaOH combines with the hydrogen ion (\(H^+\)) from acetic acid (HAc) to form water (H\(_2\)O), while the acetate ion (\(Ac^-\)) is produced as a result.
The other options listed do not accurately represent the reaction that occurs when NaOH is added to the buffer solution.
Therefore, the correct answer is option c.
Learn more on buffer solutions:
https://brainly.com/question/8676275
#SPJ11
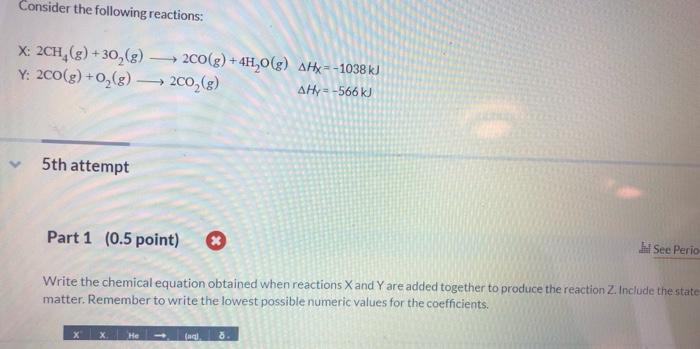
write the chemical equation obtained when reactions x and y are added together to produce the reaction z. include the states of matter. remember to write the lowest possible numeric values for the coefficients.
Answers
When we add the two reactions X and Y as shown, we obtain the reaction Z; 2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
From the full question, we can see that reaction X is given as;
2CH4(g) +3O2(g) →2CO(g) + 4H2O(g)
Reaction Y is given as;
2CO(g) + O2(g) → 2CO2(g)
If we add the two reactions together, we have;
2CH4(g) +3O2(g) + 2CO(g) + O2(g) → 2CO(g) + 4H2O(g) + 2CO2(g)
We now have to cancel out 2CO(g) on both sides of the reaction equation;
2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
Hence the reaction Z is 2CH4(g) +3O2(g) + O2(g) → 4H2O(g) + 2CO2(g)
Learn more: https://brainly.com/question/14281129
THIS IS FOR SCIENCE PLS HELP ME WRITE MY PROMPT How does the human population and human activity affect Earth?
Answers
to the satisfaction of virtually all scientists, the "mystery" of who had built the mounds in the american midwest and southeast was solved
Answers
Answer: It is not accurate to claim that the "mystery" of who had built the mounds in the American Midwest and Southeast has been completely solved to the satisfaction of virtually all scientists.
Explanation:
While there has been extensive research and progress made in understanding these ancient mounds, there are still debates and ongoing investigations surrounding their origins and the cultures responsible for their construction.
The mounds, such as those found at Cahokia in Illinois or the various mound sites in Ohio, were built by indigenous peoples of North America. However, determining specific details about the builders, their identities, and the reasons behind mound construction can be challenging due to limited historical records and the passage of time.
Archaeologists and anthropologists have used various methods, including radiocarbon dating, artifact analysis, and excavation techniques, to study these sites. They have identified different mound-building cultures such as the Adena, Hopewell, and Mississippian cultures. Still, there are unanswered questions and ongoing research to refine our understanding of these ancient civilizations and their mound-building practices.
While progress has been made in unraveling the mysteries surrounding the mound builders of the American Midwest and Southeast, it would be inaccurate to claim that the matter has been definitively resolved and satisfies all scientists. Research and exploration in this field continue to shed light on these fascinating ancient cultures and their monumental constructions.
Learn more about American mounds here, https://brainly.com/question/18296863
#SPJ11
Why do you think Leeuwenhoek was so excited about what he saw?
Answers
Answer:
van Leeuwenhoek called the organisms he saw in his microscope "animalcules." also he shined light on specimen so that he could see the right cells
Right before a predicted overnight freeze, farmers spray water on crops to protect the plants. Use the properties of water to explain how this method works. Be sure to identify why hydrogen bonds are responsible for this phenomenon.
Answers
Plants are sprayed with water at night to absorb the heat of freezing thereby keeping the plant warm and avoiding the overnight freeze.
How to avoid overnight freezeOne of the concerns that farmers have during winter is hot to keep their crops from freezing. When the crop freezes, yield of the crop could be affected in the long run.
It is common to spray the plant with water before night so that it can take away the heat of freezing thereby keeping the plant warm and avoiding the overnight freeze.
Learn more about overnight freeze: https://brainly.com/question/12703377?
. The density of gold is 19.32 g/mL. A U.S. $5.00 gold coin has a diameter of 1.62 cm and a thickness of 0.085 cm. The price of gold is currently $53.90 per gram. What is the value of the gold in the coin. V = π r2 h.
Answers
Answer:
$182 is the value of the gold in the coin
Explanation:
Diameter is 2r, the ratio of the coin is = 1.62cm / 2 = 0.81cm
Following the formula of the volume of the coin:
V = π r² h
V = π*(0.81cm)²*0.085cm
V = 0.172cm³ = mL
As the density of the gold is 19.32g/mL, the mass of 0.1752mL of gold is:
0.1752mL * (19.32g / mL) =
3.385g is the mass of the coin
As the price of the gold is $53.90 / g, the value of the gold in the coin is:
3.385g * ($53.90 / g) =
$182 is the value of the gold in the coin