the smallest group of particles within a crystal that retains the shape of the crystal
Answers
The smallest group of particles within a crystal that retains the shape of the crystal is called a unit cell.
A crystal lattice is a three-dimensional arrangement of atoms, ions, or molecules in a repeating pattern that extends throughout the material. The unit cell is the basic repeating unit of the crystal lattice. It is the smallest part of the crystal that, when repeated in three dimensions, creates the entire crystal.
The unit cell is defined by its edges, angles, and the positions of its constituent atoms, ions, or molecules. The different types of unit cells, such as simple cubic, body-centered cubic, and face-centered cubic, are distinguished by their edge lengths, angles, and the positions of their constituent particles. Understanding the unit cell is essential for understanding the physical and chemical properties of materials, such as their mechanical, electrical, and optical properties.
The complete question is
The smallest group of particles within a crystal that retains the shape of the crystal is?
To know more about the Crystal, here
https://brainly.com/question/31985497
#SPJ4
Related Questions
Karina strikes a match to light a candle
Answers
Answer:
A burning match represents an exothermic reaction. The chemicals release energy in the form of heat and light as the reaction progresses.
Explanation:
Sample Response
What is the frequency of radiation whose wavelength is 4.50 × 10^-5 cm?
Answers
The frequency of radiation is directly proportional to its wavelength. To find the frequency of radiation with a given wavelength, we can use the following formula:
frequency = speed of light / wavelength
where the speed of light is approximately 3.0 x 10^8 meters per second.
In this case, the frequency of radiation with a wavelength of 4.50 x 10^-5 cm is:
frequency = 3.0 x 10^8 m/s / (4.50 x 10^-5 cm)
= 3.0 x 10^8 m/s / (4.50 x 10^-5 x 10^-2 m/cm)
= 6.67 x 10^13 Hz
The frequency of radiation with a wavelength of 4.50 x 10^-5 cm is approximately 6.67 x 10^13 Hz.
write the formula of three compounds which you know and name the elements in them
Answers
Answer:
\(\boxed{\sf{view \ explanation}}\)
Explanation:
Compound definition:
A compound is a chemical substance formed by two or more chemically bonded elements.
Three compounds:
Water is a compound with the formula \(\sf H_2O\).
Two hydrogen atoms and one oxygen atom is present in one molecule of water.
Sodium chloride or table salt is a compound with the formula \(\sf NaCl\).
One sodium atom and one chlorine atom is present in one molecule of sodium chloride.
Ammonia is a compound with the formula \(\sf NH_3\).
In one molecule of ammonia, one nitrogen atom and three hydrogen atoms are present.
Answer:
See below
Explanation:
Three compounds are:
1) \(\mathrm {H_{2}SO{4}}\) [Sulfuric acid]
The elements in this compound are hydrogen (H) , Sulfur (S) and Oxygen (O).
2) \(\mathrm {NaOH}\) [Sodium hydroxide]
The elements in this compound are Sodium (Na) , Oxygen (O) and Hydrogen (H).
3) \(\mathrm {HCl}\) [Hydrochloric acid]
The elements are Hydrogen (H) and Chlorine (Cl).
I really need help on this question! Thank you so much!

Answers
Answer:
A. Empirical formula => C₂H₄O
B. Molecular formula => C₄H₈O₂
Explanation:
From the question given above, the following data were obtained:
Mass of C = 0.273 g
Mass of H = 0.046 g
Mass of O = 0.182 g
Molar mass of compound = 88 g/mol
A. Determination of the empirical formula of the compound.
C = 0.273 g
H = 0.046 g
O = 0.182 g
Divide by their molar mass
C = 0.273 /12 = 0.023
H = 0.046 /1 = 0.046
O = 0.182 /16 = 0.011
Divide by the smallest
C = 0.023 / 0.011 = 2
H = 0.046 / 0.011 = 4
O = 0.011 / 0.011 = 1
Thus, the empirical formula of the compound is C₂H₄O
B. Determination of the molecular formula of the compound.
Empirical formula = C₂H₄O
Molar mass of compound = 88 g/mol
Molecular formula =?
Molecular formula = [C₂H₄O]ₙ
[C₂H₄O]ₙ = 88
[(12×2) + (4×1) + 16]n = 88
[24 + 4 + 16]n = 88
44n = 88
Divide both side by 44
n = 88 / 44
n = 2
Molecular formula = [C₂H₄O]ₙ
Molecular formula = [C₂H₄O]₂
Molecular formula = C₄H₈O₂
which statmemt is true for most chemical reactions an energy change occurs during the breaking and forming of bonds
Answers
An energy change occurs during the breaking and forming of bonds is true for most chemical reactions an energy change occurs during the breaking and forming of bonds .
Chemical bonds between atoms and molecules can break and form during chemical reactions. Existing bonds must be broken in order for new ones to form, which releases energy during the process. The enthalpy change or overall energy change during a chemical reaction is typically expressed as the energy difference between the products and the reactants.
Depending on whether energy is absorbed or released during a reaction, the enthalpy change can either be positive or negative. The reaction is exothermic and energy is released if the enthalpy of the products is lower than the enthalpy of the reactants. The reaction is endothermic and energy is absorbed if the enthalpy of the products is greater than the enthalpy of the reactants.
The question is incomplete, complete question will be "Which statement is true for most chemical reactions?
An energy change occurs during the breaking and forming of bonds.
The internal energy of the system increases during a reaction.
Energy is released during the formation of reactants.
The enthalpy of the products is higher than the enthalpy of the reactants."
Learn more about chemical reactions at:
brainly.com/question/29762834
#SPJ1
You are conducting an experiment in science class. You fill Beaker 1 with 75 mL of water. You fill beaker 2 with 25 mL of water. They both are at the same temperature.
Which one has more thermal energy and why?
Answers
The Beaker of water that has more thermal energy is Beaker 1.
How to find the beaker with more thermal energy ?The total energy of all the particles is known as thermal energy. This implies that larger objects with slower-moving particles and lower temperatures can have more energy than smaller ones with higher temperatures (faster moving particles).
The amount of material in a sample of matter is measured by mass, hence the thermal energy is a function of the measured object's mass and temperature. An object's mass determines how many particles it contains and how much thermal energy it has at a given temperature.
For this reason, Beaker 1 which had more water, will have more thermal energy.
Find out more on thermal energy at https://brainly.com/question/7541718
#SPJ1
The most reactive metals are located in which area of the periodic table?
a. top
b. far left
c. far right
d. center
e. potassium, chromium, calcium
Answers
"The most reactive metals are typically located in the far left area of the periodic table, also known as the alkali metals." These metals include elements like lithium, sodium, and potassium.
Alkali metals are highly reactive because they have only one electron in their outermost energy level, which they readily lose to form positive ions. Key characteristics of alkali metals include:
1. Reactivity: Alkali metals are the most reactive metals. They readily lose their outermost electron to form a +1 ion, making them highly reactive with other elements.
2. Softness: Alkali metals have low hardness and can be easily cut with a knife.
3. Low density: They have low densities compared to other metals.
4. Low melting and boiling points: Alkali metals have relatively low melting and boiling points.
5. Good conductors of heat and electricity: They are efficient conductors of heat and electricity.
6. Reactivity with water: Alkali metals react vigorously with water, producing hydrogen gas and hydroxide ions.
7. Oxidation: Alkali metals readily react with oxygen in the air, forming oxides or peroxides.
8. Flame coloration: Alkali metals, when heated, produce distinct colors in flames. For example, sodium imparts a yellow color, and potassium gives a lilac color.
Alkali metals are important in various applications, such as batteries, alloys, and certain chemical reactions. However, their high reactivity makes them challenging to handle safely, requiring special precautions due to their tendency to react explosively with moisture or air.
To know more alkali metals visit:
https://brainly.com/question/15220625
#SPJ11
what is the source of the electrons that replace those lost by chlorophyll a in the photosystems?
Answers
The source of electrons that replace those lost by chlorophyll a in the photosystems is water molecules.
During photosynthesis, light energy is used to split water molecules into oxygen, hydrogen ions, and electrons. The electrons are then transferred to chlorophyll a in the photosystems, replacing the electrons that were lost during the initial absorption of light.
This process, known as photolysis, provides the necessary electrons for the photosystems to continue the process of electron transport and ultimately produce ATP and NADPH for the Calvin cycle. Without the continuous supply of electrons from water, photosynthesis would not be able to occur.
More on photosynthesis: https://brainly.com/question/29764662
#SPJ11
an enzyme-catalyzed reaction was carried out with the substrate concentration initially a thousand times greater than the km for that substrate. after 15 minutes, 1% of the substrate had been converted to product, and the amount of product formed in the reaction mixture was 25 mmol. if, in a separate experiment, one-fourth as much enzyme and twice as much substrate had been combined, how long would it take for the same amount (25 mmol) of product to be formed?
Answers
In the second experiment, it would take 60 minutes to produce the same amount of product (25 mmol).
We can start by using the Michaelis-Menten equation to determine the initial reaction rate in the first experiment, where the substrate concentration is initially a thousand times greater than the KM:
v₀ = Vmax × [S]/(KM + [S])
Since the substrate concentration is initially a thousand times greater than the KM, we can assume that [S] >> KM, and simplify the equation to:
v₀ = Vmax × [S] / KM
The amount of substrate converted to product after 15 minutes is 1% of the initial substrate concentration, so the remaining substrate concentration is 99% of the initial concentration. Let's define [S]0 as the initial substrate concentration, then:
[S] = 0.99 [S]₀
The amount of product formed in 15 minutes is 25 mmol, so we can calculate the initial reaction rate as:
v₀ = (25 mmol / 15 min) / (0.01 mol / mol) = 1666.7 nmol/min
where we have converted the units to nmol/min and used the molecular weight of the product to convert from moles to millimoles.
Now, let's consider the second experiment, where one-fourth as much enzyme and twice as much substrate are used.
Since the amount of product formed is the same in both experiments, we can write:
v₁ × t₁ = v₂ × t₂
where v₁ and t₁ are the initial reaction rate and reaction time in the first experiment, and v₂ and t₂ are the corresponding values in the second experiment.
Let's use the Michaelis-Menten equation to relate the initial reaction rate to the enzyme and substrate concentrations:
v₁ = Vmax × [S]₁ / (KM + [S]₁) × E₁
v₂ = Vmax × [S]₂ / (KM + [S]₂) × E₂
We can rearrange these equations to solve for [S]1 and [S]2 in terms of v1, v₂, E₁, E₂, and KM:
[S]₁ = (v₁ × KM) / (Vmax × E₁) - [S]₂
[S]₂ = (v₂ × KM) / (Vmax × E₂) - [S]₁
Substituting these expressions into the equation for the reaction time, we get:
t₂ = (v₁ × t₁ × Vmax × E₂) / (v₂ × Vmax × E₁ + v₁ × E₂ × KM / (Vmax × E₁) - v₁ × KM / (Vmax × E₁))
Substituting the values from the first experiment, we get:
t₂ = (1666.7 nmol/min × 15 min × 1/4 × 2) / (1666.7 nmol/min × 1/2 × 1/1000 mol/L + 1666.7 nmol/min × 2 × 0.01 mol/L / (1/1000 mol/L) - 1666.7 nmol/min × 0.01 mol/L / (1/1000 mol/L))
Simplifying, we get:
t₂ = 60 min
To know more about Michaelis-Menten equation here
https://brainly.com/question/20912949
#SPJ4
OML HELP MEEEEEE PLEASEEEEE!!!!!!!!!!
Why might there be an advantage for flowers that attract only one type of pollinator and exclude others? What would be the disadvantage? Explain your reasoning.
Answers
(omg, your in high school, but I'm in middle school but I have this same question lol.)
There would be an advantage for flowers that attract only one type of pollinator and excude others because the flower could be harmed by the pollinator. The flower could have a natural defense against certain species of animals to avoid things like getting completely eaten or getting a virus from an infected species. The disadvantage would be that some species that the flower needs might avoid the flower because of natural defense.
I hope this helps! Sorry if it doesn't because I'm only in middle school.
If a plant has a specific pollinator, a good relationship evolves between the plant and the insect. If the plant becomes extinct, the plant also becomes extinct.
Pollination is the process by which pollen grains are transferred from the anther to the stigma of a flower. It is normally carried out by the agents of pollination such as wind, insects etc.
If a flower only has a specific pollinator, it is much easier for the plant to be pollinated because the pollinator evolves a good relationship with the plant.
However, if that specific pollinator happens to become extinct, that plant also becomes extinct because there will be no other pollinator for the plant specie.
Learn more about pollination: https://brainly.com/question/1675149
please help with my chemistry homework thank you so much

Answers
Answer:
There are 2.2 moles of hydrogen.
Explanation:
The given information from the exercise is:
- Volume (V): 48.6L
- Temperature (T): 0°C (273K)
- Pressure (P): 1atm
1st) It is important to convert the temperature unit from °C to Kelvin:
\(0+273=273K\)This first step is very important, because all the units must be at atm, L and Kelvin, before using the Ideal Gases Law formula.
2nd) Now to calculate the moles of hydrogen, we hae to replace the values of V, T and P in the Ideal Gases Law formula:
\(\begin{gathered} P*V=n*R*T \\ 1atm*48.6L=n*0.082\frac{atm*L}{mol*K}*273K \\ 48.6atm*L=n*22.4\frac{atm*L}{mol} \\ \frac{48.6atm}{22.4\frac{atm*L}{mol}}=n \\ 2.2moles=n \end{gathered}\)R: is the gases constant, in its value is 0.082atm.L/mol.K.
So, there are 2.2 moles of hydrogen.
using your potentiometric titration data, calculate the molar concentration of the original silver nitrate (agno3) solution.
Answers
Potentiometric titration data, the molar concentration of the original silver nitrate (AgNO₃) solution is 0.1015 M.
The potentiometric data is given as follows :
molar concentration NaCl, M1 = 0.1026 M
volume of the NaCl = 9.90 mL
volume of the silver nitrate, AgNO₃ = 10 mL
molar concentration of silver nitrate = ?
M1 V1 = M2 V2
where,
Molarity M1 = 0.1026 M
volume V1 = 9.90 mL
volume V2 = 10 mL
molarity M2 = ?
0.1026 × 9.90 = M2 × 10
M2 = 0.1015 M
Thus, using Potentiometric titration data, the molar concentration of the original silver nitrate (AgNO₃) solution is 0.1015 M.
To learn more about molarity here
https://brainly.com/question/20528613
#SPJ4
The polar ethanol solvent in the Sn1 experiment with AgNO3 is optimal for unimolecular reactions because it is _ the carbocation intermediate. aprotic, stabilizes O protic, stabilizes O aprotic; destabilizes O protic, destabilizes
Answers
The polar ethanol solvent in the Sn1 experiment with \(AgNO_{3}\) is optimal for unimolecular reactions because it is protic and stabilizes the carbocation intermediate.
Why are polar solvents used in Sn1 reaction?
The polar ethanol solvent in the Sn1 experiment with \(AgNO_{3}\) is optimal for unimolecular reactions because it is protic and stabilizes the carbocation intermediate. Protic solvents, like ethanol, have hydrogen atoms bonded to electronegative atoms, which allows them to form hydrogen bonds with the carbocation intermediate, stabilizing it and facilitating the Sn1 reaction.
Aprotic solvents, on the other hand, lack these hydrogen atoms and can actually destabilize the carbocation intermediate, leading to rearrangements or other unwanted reactions. Therefore, the use of polar ethanol solvent in the Sn1 experiment with \(AgNO_{3}\) is beneficial for the formation of a stable carbocation intermediate and the success of the reaction.
To know more about Sn1 reaction:
https://brainly.com/question/29589832
#SPJ11
14.
How many grams of copper are in 4.6 moles of copper?
Answers
Answer:
292.1 kg = 292 100 g
Explanation:
n = m/Mm
Mm(Cu) = 63.5
m = n * Mm
m = 4.6 * 63.5
m = 292.1 kg
a scale model of the solar system
Answers

Which two events will happen if more H2 and N2 are added to this reaction after it reaches equilibrium?
3H2 + N2 to 2NH3
Answers
If more \(H_{2}\) and \(N_{2}\) are added to the reaction 3\(H_{2}\) + N2 → 2\(NH_{3}\) after it reaches equilibrium, two events will occur Shift in Equilibrium and Increased Yield of \(NH_{3}\)
1. Shift in Equilibrium: According to Le Chatelier's principle, when additional reactants are added, the equilibrium will shift in the forward direction to consume the added reactants and establish a new equilibrium. In this case, more \(NH_{3}\) will be produced to counteract the increase in \(H_{2}\) and \(N_{2}\).
2. Increased Yield of \(NH_{3}\): The shift in equilibrium towards the forward reaction will result in an increased yield of \(NH_{3}\). As more \(H_{2}\) and \(N_{2}\) are added, the reaction will favor the production of \(NH_{3}\) to maintain equilibrium. This will lead to an increase in the concentration of \(NH_{3}\) compared to the initial equilibrium state.
It is important to note that the equilibrium position will ultimately depend on factors such as the concentrations of \(H_{2}\), \(N_{2}\), and \(NH_{3}\), as well as the temperature and pressure of the system. By adding more reactants, the equilibrium will adjust to achieve a new balance, favoring the formation of more \(NH_{3}\).
Know more about Le Chatelier's principle here:
https://brainly.com/question/2943338
#SPJ8
What does the atomic number of an element represent?
A. number of isotopes
B. number of protons
C. number of bonds
D. number of atoms
Answers
Answer:
protons
Explanation:
btw you protons and electrons are always the same
prcAnswer:
e c. proton
Explanation:
i pueslist
a solution naoh(aq)naoh(aq) contains 7.17.1 g naoh(s)naoh(s) per 100.0100.0 ml of solution. calculate the ph and the poh of the solution at 2525 °c.
Answers
At 25°C, the pH of the solution is approximately 14.249 and the pOH is approximately -0.249.
The question asks for the pH and pOH of a solution containing NaOH at 25°C.
To calculate the pH and pOH, we first need to determine the concentration of hydroxide ions (OH-) in the solution.
Given that the solution contains 7.1 g of NaOH per 100.0 ml of solution, we need to convert the mass of NaOH to moles.
The molar mass of NaOH is 22.99 g/mol (Na) + 16.00 g/mol (O) + 1.01 g/mol (H) = 39.99 g/mol.
Using the molar mass, we can calculate the number of moles of NaOH:
7.1 g NaOH * (1 mol NaOH / 39.99 g NaOH) = 0.1775 mol NaOH
Now, we need to calculate the concentration of hydroxide ions (OH-) in the solution.
The volume of the solution is given as 100.0 ml, which is equivalent to 0.1000 L.
Concentration (C) is calculated by dividing the number of moles (n) by the volume (V) in liters:
C = n / V
C(OH-) = 0.1775 mol NaOH / 0.1000 L = 1.775 mol/L
Now, we can calculate the pOH using the formula:
pOH = -log10(C(OH-))
pOH = -log10(1.775) ≈ -0.249
To find the pH, we can use the fact that pH + pOH = 14 at 25°C. pH = 14 - pOH = 14 - (-0.249) = 14.249.
To know more about pH and pOH visit:
brainly.com/question/8896097
#SPJ11
arrange the four liquids in the order they would position themselves in a test tube based on their densities.
Answers
The order of the four liquids in the test tube based on their densities, from top to bottom, is as follows: Oil, Water, Honey, and Mercury.
In a test tube, the liquids will arrange themselves in order of increasing density. The least dense liquid will float on top, while the denser liquids will sink below. The first liquid, oil, has the lowest density and will float on top. Next, water, with a higher density, will settle beneath the oil.
Following water, honey, which is denser than water, will sink further down. Lastly, mercury, being the densest of the four liquids, will settle at the bottom of the test tube. This arrangement is a result of the liquids' different densities, with the denser liquids displacing the less dense ones.
To learn more about densities, click here:
brainly.com/question/15164682
#SPJ11
Calculate the mass of N in 2.34 g of N2H4?A) 4.68 g N B) 65.6 g N C) 28.02 g N D) 2.05 g N E) 2.34 g N
Answers
D) 2.05 g N. The molar mass of N2H4 is 32.045 g/mol. To calculate the mass of N in 2.34 g of N2H4, we need to first calculate the number of moles of N2H4:
moles of N2H4 = (mass of N2H4) / (molar mass of N2H4)
moles of N2H4 = 2.34 g / 32.045 g/mol
moles of N2H4 = 0.073 mol
Since there are two N atoms in one N2H4 molecule, we need to multiply the number of moles of N2H4 by 2 to get the number of moles of N:
moles of N = 2 x moles of N2H4
moles of N = 2 x 0.073 mol
moles of N = 0.146 mol
Finally, we can calculate the mass of N:
mass of N = (moles of N) x (molar mass of N)
mass of N = 0.146 mol x 14.007 g/mol
mass of N = 2.05 g
Find out more about molar mass
brainly.com/question/30892478
#SPJ11
A buffer that contains 0.54 M of the base, B, and 0.13 M of its conjugate acid, BH+, has a pH of 8.80. What is the pH after 0.0039mol HCl is added to 0.25 L of this solution?
Answers
The pH after adding 0.0039 mol of HCl to the buffer solution is approximately 8.59.
After adding 0.0039 mol of HCl to 0.25 L of a buffer solution containing 0.54 M base (B) and 0.13 M conjugate acid (BH+), with an initial pH of 8.80, the new pH can be calculated using the Henderson-Hasselbalch equation:
pH = pKa + log([A-]/[HA])
First, we need to find the pKa using the initial pH, base, and conjugate acid concentrations:
8.80 = pKa + log(0.54/0.13)
pKa ≈ 7.93
Next, calculate the moles of base (B) and conjugate acid (BH+) in the buffer solution:
moles of B = 0.54 M * 0.25 L = 0.135 mol
moles of BH+ = 0.13 M * 0.25 L = 0.0325 mol
Now, consider the reaction between HCl and the base (B):
B + HCl → BH+
moles of HCl added = 0.0039 mol
After the reaction, the moles of base (B) and conjugate acid (BH+) will change:
moles of B = 0.135 - 0.0039 = 0.1311 mol
moles of BH+ = 0.0325 + 0.0039 = 0.0364 mol
Finally, calculate the new pH using the Henderson-Hasselbalch equation:
new pH = 7.93 + log(0.1311/0.0364) ≈ 8.59
So, the pH after adding 0.0039 mol of HCl to the buffer solution is approximately 8.59.
Learn more about acid-base chemistry and buffer solutions :https://brainly.com/question/27371101
#SPJ11
Plz plz here
A student pours 25.0 mL of water into a graduated cylinder. She drops a plece of unknown metal with a mass of 18.9 g Into the cylinder. The
water level rises to 32.0 mL. The student uses the table to identify the metal
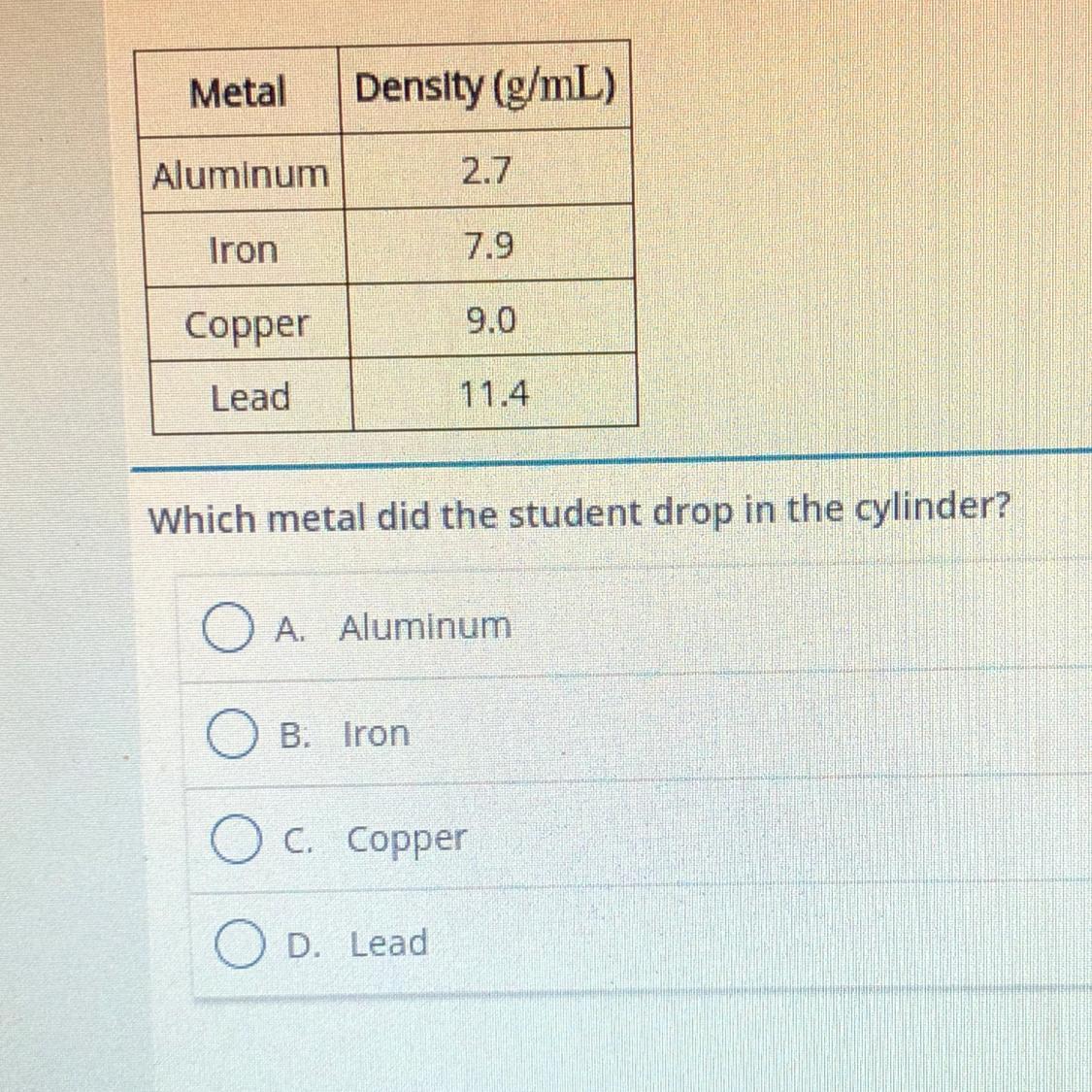
Answers
Answer:
Aluminum
Explanation
The volume of the metal is found by subtracting the final volume by the Intel volume giving 7
And the formula for density is mass divided by volume hence minus 7 by 18.9 giving 2.7
C3Hg has a higher lower boiling point than C₂H6-
Answers
Answer:
C₃H₈ has a higher boiling point than C₂H₆
Explanation:
The boiling point is the temperature at which the intermolecular forces between molecules are overcome and the state of the substance changes from liquid to gas.
Since these are both hydrocarbons (molecules containing just carbon and hydrogen), the strength of their intermolecular bonds should be the same. However, since C₃H₈ is larger, it can form more of these intermolecular bonds. Thus, it takes more energy and a higher temperature to overcome the increased amount of forces.
If the electronnegavity difference between elements A and X is 0.8, the bond in AX will most likely be what?
Answers
Answer:
Polar covalent bond
Explanation:
When the electronegativity difference between two elements A and X is 0.8, the bond AX formed will most likely be a polar covalent bond.
A polar covalent bond is one whose electronegativity difference is between 0.5 and 2.1.
In such a bond type, we have heteronuclear species with one of the species having a higher electronegativity value.
When electronegativity difference is less than 0.5, a non-polar covalent bond forms. If the value is greater than 2.1, an ionic bond will form.use unit cancellation method to solve the following problems 51 mi/hr to km/hr
Answers
To use the unit cancellation method to solve this problem, we will need to know the conversion factor between miles per hour (mi/hr) and kilometers per hour (km/hr). This conversion factor is 1.60934 km/hr for every 1 mi/hr.
Now, we can set up our problem and use the unit cancellation method to solve the answer:
51 mi/hr * 1.60934 km/hr/1 mi/hr = 82.077 km/hr
To solve this problem, we started with the given speed in miles per hour (51 mi/hr). Then, we multiplied it by the conversion factor of 1.60934 km/hr for every 1 mi/hr. By setting up the units in the numerator and denominator to cancel out, we were left with the final answer of 82.077 km/hr.
In summary, using the unit cancellation method is a useful tool for converting units and solving problems. By setting up the units in a way that cancels out, we can easily and accurately convert between different units.
For more information on unit cancellation method visit:
brainly.com/question/29098308
#SPJ11
How many electrons does calcium donate to sulfur when calcium and sulfur form an ionic bond?
Answers
Answer:
two valence electrons
Explanation:
Use the periodic to fill in the numbers in the electron configurations shown below.
B: 1s2 2sA2pB
A =
2
B =
1
Na: 1s22sC2pD3sE
C =
D =
E =
Answers
Answer:
B: 1s²2s²2p¹
Na: 1s²2s²2p⁶3s¹
Explanation:
A = 2
B = 1
C = 2
D = 6
E = 1
Why are xrays used to probe the crystal structure of a material
Answers
Answer:
Because X-rays have wavelengths similar to the size of atoms
X rays are used to probe the crystal structure of a material because this have same wavelength as atoms of crystal have.
What are x rays?X rays are the type of electromagnetic radiations and it is produced when the fast moving electron collide with the target anode and sudden deceleration takes place.
X rays have the high energy so that it easily penetrate in any crystal molecule as they also have same wavelength as atoms of the crystals have.
Hence due to high energy and same wavelength was of atoms of crystal it is used to probe the crystal.
To know more about x rays, visit the below link:
https://brainly.com/question/11305216
#SPJ4
An invasive species is a species that disrupts the ecosystem into which it is introduced by displacing species.
Answers
An existing ecosystem can suffer from damage from invasive species in many different ways, such as shifting habitats and depriving native creatures of food and resources.
What is an invasive species?An invasive species is an organism that damages the environment or the economy in a new location in which it is not native. Invasive species may disrupt the food chain of a ecosystem by killing or substituting natural food sources. The invasive species could provide little to no food value to wildlife.
What is an example of a invasive species?Accidental introduction of invasive species into a new area is common. In Central Asia, the Black Sea and the Caspian Sea are home to zebra mussels. Zebra mussels accidently made their way to the Great Lakes of North America after becoming affixed to massive ships that sailed between the two areas.
To know more about Invasive species visit:
https://brainly.com/question/21452505
#SPJ9
Newton's second law of motion described the relationship among force, mass, and acceleration. Write the equation.
Please help me vote you brainiest
Answers
Answer:
Second Law of Motion (Law of acceleration)
- It states that the acceleration of an object equals the net force acting on the object divided by the object's mass.
To simplify, use the magic formula triangle
the units of these quantities were as follows: Force (Kilogram meter per second squared or kg m/s² which is also equivalent to one Newton, or N), mass (kilogram or kg), and acceleration (meter per second squared or m/s²).
The Formula on Law of acceleration is:
If the force is missing, Force = Mass × Acceleration or F = ma
If the mass is missing, Mass = Force ÷ Acceleration or m = F/a
If the acceleration is missing, Acceleration = Force ÷ Mass or a = F/m