The tectonic plates float on the lower portion of Earth's crust?
Answers
Answer:
The tectonic plates comprise the bottom of the crust and the top of the Earth's mantle. There are ten major plates on Earth and many more minor ones. They float on a plastic-like part of the Earth's mantle called the asthenosphere.
Explanation:
Answer:
Yes
Explanation:
Related Questions
7. A dog sled is pulled by 8 dogs and accelerates at 1.2 m/s². If each dog pulls with a force of 30 N, what
is the combined mass of the sled and rider?
Answers
The combine mass of the sled and the rider, given that each dog pulled with a force of 30 N is 200 Kg
How to determine the combine massWe know that force is related to mass and acceleration according to the following formula:
Force (F) = mass (m) × acceleration (a)
F = ma
With the above formula, we can determine the combined mass of the sled and rider. Details below.
From the question given above, the following data were obtained:
Acceleration (a) = 1.2 m/s²Force of each dog = 30 NForce of 8 dogs = 8 × 30 = 240 NCombined mass (m) =?The combined mass can be obtained as follow:
Force = mass × acceleration
240 = mass × 1.2
Divide both sides by 1.2
Mass = 240 / 1.2
Mass = 200 Kg
Thus, the combine mass is 200 Kg
Learn more about force, mass and acceleration:
https://brainly.com/question/12185838
#SPJ1
does an identical cylinder with the same pressure of hydrogen contain more molecules than a cylinder of oxygen because hydrogen molescules are smaller
Answers
An identical cylinder containing hydrogen at the same pressure as a cylinder containing oxygen will contain more hydrogen molecules because hydrogen gas (H2) has a lower molar mass than oxygen gas (O2). This means that there are more H2 molecules in a given volume at a given pressure, as compared to O2.
Because hydrogen gas (H2) has a smaller molar mass than oxygen gas, an identical cylinder holding hydrogen at the same pressure will hold more hydrogen molecules (O2). This indicates that compared to O2, there are more H2 molecules in a given volume at a given pressure.
Learn more about identical cylinder here:
https://brainly.com/question/28474923
#SPJ4
A student proposes the following step of a mechanism. Why would an expert question this mechanism step? 3A+B→2C A) The number of reactants and products must be the same. B) The number of products must always exceed the reactants. C) This would require 4 molecules to collide and react simultaneously.
Answers
Option C. An expert would question the proposed mechanism step 3A+B→2C due to the requirement of four molecules to collide and react simultaneously.
The expert would question this mechanism step for several reasons. Firstly, according to the law of conservation of mass, the number of atoms of each element must be the same on both sides of a chemical equation. In the proposed step, there are three reactant molecules (3A and B) but only two product molecules (2C), violating the principle that the number of reactants and products must be the same.
Secondly, the statement that the number of products must always exceed the number of reactants is incorrect. While it is possible for the number of products to exceed the number of reactants in some chemical reactions, it is not a universal rule. There are reactions where the number of products is equal to or even less than the number of reactants.
Finally, the mechanism step suggests that four molecules (3A and B) would need to collide and react simultaneously, which is highly unlikely. In most chemical reactions, collisions between molecules occur randomly, and it is rare for four molecules to collide at the exact same time and in the correct orientation.
Learn more about reactants here:
https://brainly.com/question/30129541
#SPJ11
For the reaction C+2H 2 —->CH 4 calculate the percent yield if 98 g of methane is produced when 100. g of carbon reacts with an excess of hydrogen?
Answers
The percent yield : 73.5%
Further explanationGiven
Reaction
C+2H₂⇒CH₄
Required
The percent yield
Solution
mol of Carbon(as a limiting reactant) :
\(\tt \dfrac{100}{12}=8.3\)
mol CH₄ based on C, and from equation mol ratio C : CH₄, so mol CH₄ = 8.3
Mass of Methane(theoretical yield) :
\(\tt mass=mol\times MW\\\\mass=8.3\times 16=133.3~g\)
\(\tt \%~yield=\dfrac{actual}{theoretical}\times 100\%\\\\\%yield=\dfrac{98}{133.3}\times 100\%=73.5\%\)
Which correctly describe a reversible reaction reaching equilibrium in a closed system?
Select two that apply.
-Over time, the rates of the forward and reverse reactions equalize.
-Over time, the rate of the forward reaction becomes zero.
-Initially, the concentration of reactants is low, so the rate of the forward reaction is also low.
-Initially, the concentration of products is low, so the rate of the reverse reaction is also low.
-Over time, the rate of the reverse reaction becomes greater than the forward reaction.
Answers
Answer:
-Over time, the rates of the forward and reverse reactions equalize.
-Initially, the concentration of products is low, so the rate of the reverse reaction is also low.
Explanation:
A chemical reaction is said to be reversible when the reactants forms the products, which in turn reacts together again to give rise to the reactants. In a reversible reaction, the formation of products from reactants occurs simultaneously with the reformation of the reactants from the products. For example:
The reversible reaction: A + B ⇆ C + D means;
A + B → C + D and C + D → A + B
The rate at which both forward and reverse reactions are taking place in closed system may be initially different but with time, it gets equal to form an equilibrium reaction. However, at first, only the rate of the forward reaction proceeds because the concentration of the product is low. Hence, the rate of reaction of the reverse reaction (product to reactants) is low as well.
In the reversible reaction above, the rate of the reverse reaction (C + D → A + B) will turn out low initially because the concentration of the products (C and D) are low. With time, the rates of the forward and reverse reaction becomes equal to form an EQUILIBRIUM or STABLE reaction.
what is the h 3o concentration in 0.0072 m naoh(aq) at 25 °c? ( k w = 1.01 × 10 –14) a. 1.4 × 10–12 m b. 1.0 × 10–14 m c. 7.2 × 10–17 m d. 7.2 × 10–3 m e. 1.0 × 10–7 m
Answers
The concentration of h 3o is 1.4 × 10–12 m hence correct answer is A
To find the H3O+ concentration in a solution of NaOH, we need to use the following equation:
Kw = [H3O+][OH-]
Where Kw is the ion product constant of water, which is equal to 1.01 × 10 –14 at 25 °C.
We know that NaOH is a strong base, which means it completely dissociates in water to form Na+ and OH- ions:
NaOH → Na+ + OH-
Therefore, the concentration of OH- ions in the solution is equal to the concentration of NaOH, which is 0.0072 M.
Now we can use the Kw equation to find the H3O+ concentration:
Kw = [H3O+][OH-]
1.01 × 10 –14 = [H3O+][0.0072]
[H3O+] = 1.01 × 10 –14 / 0.0072
[H3O+] = 1.4 × 10–12 M
Learn more anout concentraion here :-
https://brainly.com/question/2201903?
#SPJ11
Flame test lab
What are the answers to the last two columns and how do I solve them?

Answers
f = 5.08 x 10¹⁴/s
E=3.366 x 10⁻¹⁹ J
Further explanationRadiation energy is absorbed by photons
The energy in one photon can be formulated as
\(\large{\boxed{\bold{E\:=\:h\:.\:f}}}\)
Where
h = Planck's constant (6,626.10⁻³⁴ Js)
f = Frequency of electromagnetic waves
f = c / λ
c = speed of light
= 3.10⁸
λ = wavelength
I will give example number 1, and for subsequent numbers, the steps will be the same
For NaNO₃ with λ=590 nm=5.9 x 10⁻⁷m
Frequency(f) :
\(\tt f=\dfrac{3.10^8}{5.9\times 10^{-7}}=5.08\times 10^{14}/s\)
Energy(E) :
\(\tt E=6.626.10^{-34}\times 5.08\times 10^{14}=3.366\times 10^{-19}~J\)
Please help me with this science quesiton

Answers
The atoms inside the reactants reorganise their chemical bonds during a chemical reaction to create products. There will therefore always be a shift in energy when chemical reactions take place.
What atomic configuration occurs throughout a chemical change?The atoms inside the reactants rearrange and link differently throughout a chemical reaction to create one or more innovative brands with properties distinct from the reactants. A chemical change occurs when a new material is created.
What chemical process causes an energy change?Exothermic refers to chemical reactions which release energy. When bonds are created in the products of exothermic processes, more energy is produced than is required to rupture the connections between the reactants. Endothermic refers to chemical reactions that either use or absorb energy.
To know more about reaction visit:
https://brainly.com/question/28984750
#SPJ1
Does ZnS,MnS,NiS and CoS (Group IV Sulphides) dissolve in dil.HCl? And I want to know the reasons
Answers
Answer:
..
Explanation:
In a significantly acidic medium, even a soluble sulfide like sodium sulfide will not facilitate precipitation of Fe, Zn, Mn, Co and Ni as their sulfides.
A scientist combines two substances and observes that the solid substance dissolves in the liquid substance, but he observes no other changes. Which statement is likely the best description of what the scientist observed?
Answers
change from solid to liquid
Explanation:
When we are on an airplane above the clouds, why does it look like we're passing up each cloud very slowly even though we're going about 500 mph?
Answers
Answer:
When we are on an airplane above the clouds, it can appear as if we're passing each cloud slowly because there is no frame of reference to judge our speed. Our eyes can't detect any movement on the ground, and there are no other objects in the sky to provide a sense of speed or motion. Additionally, clouds are often quite large, so it can take several minutes to pass over one even at high speeds. This can create the illusion that we're moving slowly, even though we're actually traveling at several hundred miles per hour. Our brains are not accustomed to seeing objects at such a high altitude and speed, so it can be difficult to accurately judge our motion relative to the clouds.
Equal but opposite forces acting on an object results in what?
Answers
Answer:
Action given and reaction taken
Also known as
Newton's third law of motion
Explanation:
An action will be done such as bouncing a ball on the wall
- You throw the ball (Action)
- The ball bounces back (Reaction)
Hope this Helps
Answer these questions to get marked as a BRAINLIEST!!!!

Answers
Answer:
c
Explanation:
Answer:
I think ur answer would be C.)
Explanation:
hope this helps <3
Which of the following is true about most predators? (Select all that apply.)
They have good hearing.
They have keen eyesight.
They are the biggest animals in the ecosystem.
They have special adaptations that help them to be successful hunters.
Answers
Answer:
select all of them except they are the biggest
The predators have good hearing, keen eyesight, and also have special adaptations that help them to be successful hunters. The correct options are A, B, and D.
What are predators?A predator is an organism that consumes all or part of the body of its prey, which is another living or recently killed organism.
"Living or recently killed" distinguishes predators from decomposers, such as fungi and bacteria, which break down the remains of dead organisms.
Predation is classified into four types: carnivory, herbivory, parasitism, and mutualism. Each type of predation can be classified based on whether or not the prey is killed.
Prey animals are prey animals that are killed and eaten by other animals. For instance, Rabbits and crickets are both prey for larger animals.
The predators have excellent hearing and vision, as well as special adaptations that allow them to be successful hunters.
Thus, the correct options are A, B, and D.
For more details regarding predators, visit:
https://brainly.com/question/28871161
#SPJ2
Do animals release water vapor to the atmosphere?.
Answers
When animals breathe out, they also emit water vapor into the atmosphere. This is referred to as respiration.
Some of the water that falls to the ground is stored by the earth. Water is excreted by animals through respiration and urine. In animals, the process is known as perspiration or sweating. Evaporation and transpiration convert liquid water into vapor, which rises into the atmosphere as air currents rise. Water vapor, also known as water vapor or aqueous vapor, is the gaseous state of water. It is a type of water in the hydrosphere.
Learn more about water vapor here:
https://brainly.com/question/22349941
#SPJ4
All of the following explains why solar energy a better source of energy except:
Solar energy produces less toxic gas in the atmosphere.
Solar energy can be mined with less damage to ecosystems.
There is less environmental impact when collecting solar energy.
Solar energy is unlimited and easily captured from the Sun.
Answers
Answer:
Solar energy can be mined with less damage to ecosystems
Explanation:
solar energy isint mined, its collected and it is kinda bad for the environment when you mine the metals to get the solar panel
Which is the most explosive element?
Answers
Answer:
Hydrogen is the most explosive element
All explosives must contain both oxidizing and reducing agents. Strong oxidizing agents require the use of the most electronegative elements nitrogen, oxygen, fluorine, and chlorine.
Hope this helps!
25 ml of a 0. 10 m solution of magnesium chloride reacts with 25 ml of potassium hydroxide to form a magnesium hydroxide precipitate. What is the minimum concentration of potassium hydroxide necessary to completely precipitate all of the magnesium?.
Answers
Magnesium chloride, often known as MgCl2, can be produced chemically by extracting it from brine or seawater.
Magnesium chloride+ potassium hydroxide (25 ml )------>magnesium hydroxide
Magnesium chloride: what is it?
One magnesium (Mg) and two chloride ions make up magnesium chloride, also known as magnesium dichloride, magnesium (II) chloride, or chloromagnesite (Cl-).
Ionic halides, such as magnesium dichloride and related salts, have the appearance of fine, white to grey granules.
It has no smell and is very water soluble.It is frequently employed as medication for numerous cellular processes.Uses of MgCl2 (Magnesium Chloride)
Magnesium metals are produced using magnesium chloride as a precursor.utilised for soil stabilisation, dust management, and wind erosion.Fire extinguishers use this.used as an additive in food.utilised in the production of paper.is a component of disinfectants.a flocculating agent is used.To learn more about Magnesium hydroxide reaction, visit
https://brainly.com/question/15287665
#SPJ13
How many mmol of iron are there in 650 mg of iron? O A. 11.6 mmol Fe B. 363.02 mmol Fe C. 55.85 mmol Fe D. 8.95 mmol Fe
Answers
There are 11.6 mmol of iron in 650 mg of iron.
Given the mass of iron as 650 mg. The molar mass of iron is 55.85 g/mol.
We need to calculate how many millimoles (mmol) are present in the given amount of iron.
We will use the following conversion:
1 g = 1000 mg
1 mol = molar mass in grams
1 mmol = 0.001 mol
Number of moles of iron
= 650 mg ÷ 1000 mg/g
= 0.65 g ÷ 55.85 g/mol
= 0.0116 mol
Number of millimoles of iron
= 0.0116 mol ÷ 0.001 mol/mmolar mass of iron
= 11.6 mmol
Hence, there are 11.6 mmol of iron in 650 mg of iron. Therefore, the correct option is A. 11.6 mmol Fe.
Learn more about the millimoles from the given link-
https://brainly.com/question/30640148
#SPJ11
What force causes a ball to move
Answers
Answer: aerodynamic force
Explanation:
The time-varying aerodynamic force causes the ball to move erratically. This motion is the source of the "dancing" knuckleball that confuses both batters and catchers alike.
The teacher was not in the room yet. Randy began opening containers of chemicals, touching them with his hands. His cheek itches so he rubbed it. Explain how Randy was not acting safety in the the laboratory.
I know the answer I just don’t know how to word it. Please help. Due by 11:59 tonight
Answers
Answer:
Randy went into the classroom without the teacher being present. He opened containers of chemicals without the teacher's consent. Randy is making contact with the chemicals on his skin.
I hope this helps! I haven't done lab science in a while so I'm trying to remember the safety precautions.
Randy went into the classroom without the teacher being present and opened containers of chemical, making contact with chemicals on skin leads to skin reaction.
So Randy should follow the lab safety rules while entering into lab.
What are the important lab safety rules?After entering lab wear apron and gloves along with safety goggles if working with any hazardous or infectious substance.
Fully aware about the hazards of the materials, properly trained to handle lab equipment, chemicals should be adequately labelled.
Taste or smell chemicals are prohibited, never pour already stored chemicals, chemical hygiene plan should be developed for specific hazards.
Avoid to adopt un-tested and unauthorized experimentation, Clean up all chemical spill, Laboratory induction should be carried out .
Avoid storage of any edible items in the refrigerator along with lab chemicals and specimens, clean the work station after completing experiment and sterilization should be done before starting experiment.
Learn more about lab safety rules , here:
https://brainly.com/question/5147285
#SPJ2
which of the following uses mechanical energy to function?
A. computer
B. battery
C. microwave oven
D. windmill
Answers
If I have a solution that consists of 1 cup of salt and 5 cups of water, if i take out 3/4 of that how much salt is in that 3/4?
Answers
Answer: 1/8
The solution is 1 part salt, 5 parts water. This means that there are 6 parts in the solution.
3/4 of 6 is 4.5 . Which means 4.5 of the 6 parts of the solution were removed from the container. 1/6 of 3/4 is .125, which is 1/8th as a fraction.
This means that 1/8th of the solution that was taken out was salt.
I hope this helped & Good Luck <3 !!!
Into how many peaks would you expect the 1H NMR signals of the indicated protons to be split? (Assume all coupling constants are equal. )
Answers
The 1H NMR signals of the indicated protons would be split into three peaks.
What are Protons?
Protons are subatomic particles that are present in the nucleus of an atom. They have a positive electric charge and are one of the three main components of an atom, along with electrons and neutrons. Protons are the heaviest of the three particles and make up a large portion of an atom's mass. The number of protons in an atom is known as its atomic number and is what determines an element's chemical behavior and properties.
The split happens because each proton is coupled to two other protons, and each proton is affected by the other two, resulting in a triplet.
To know more about protons,
https://brainly.com/question/1805828
#SPJ4
PLEASEEEEEE HELPPPP???

Answers
2. a
3. a
(I’m not 100% but 2 & 3 couldn’t be transform)
supposed chemists attempt to produce an element with atomic number 119 based on it’s likely position on the periodic table what would you expect it’s electronegativity to be? explain how you can make this prediction
Answers
An element with atomic number 119 will be an alkali metal with a +1 oxidation state which makes it highly electronegative.
What is Electronegativity?This is described as the tendency of the atom of an element to attract electrons so as to form a bond. This is done so that the elements can achieve a stable octet configuration.
On the other hand, if an element with atomic number 119 was present based on it’s likely position on the periodic table then it will most likely be an alkali metal with a +1 oxidation state and will be highly electronegative as it requires the loss of only one electron in other to achieve a stable configuration thereby making it highly reactive.
Read more about Electronegativity here https://brainly.com/question/18258838
#SPJ1
a 135 g sample of carbon disulfide requires 43.2 kj of heat to vaporize completely. what is the enthalpy of vaporization for carbon disulfide?
Answers
The enthalpy of vaporization for carbon disulfide is 0.32 kJ/g.
The enthalpy of vaporization (ΔHvap) for carbon disulfide can be calculated using the formula:
ΔHvap = q/m
Where q is the heat required to vaporize the sample and m is the mass of the sample.
Substituting the given values, we get:
ΔHvap = 43.2 kJ / 135 g
ΔHvap = 0.32 kJ/g
Therefore, the enthalpy of vaporization for carbon disulfide is 0.32 kJ/g.
Learn more about enthalpy
brainly.com/question/16720480
#SPJ11
Which of the following is the most violent of all solar disturbances?
a
solar winds
b
sunspots
c
prominence's
d
solar flares
Answers
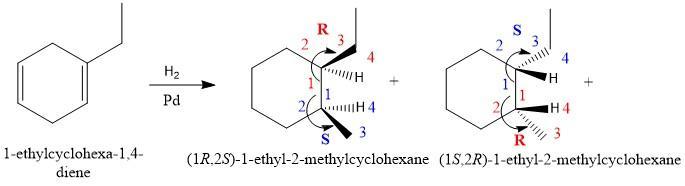
Draw the structure or structures produced by the catalytic reduction of the given compound, in which h2 is in excess. Draw hydrogen at a chirality center and use wedge‑and‑dash bonds to designate the stereochemistry, if applicable.
Answers
Structures produced by the catalytic reduction of the given compound, in which H₂ is in excess are attached as a picture.
Upon the catalytic reduction of the given compound 1-ethylcyclohexa-1,4-diene (missing in question) by using excess hydrogen, two compounds (1R,2S)-1-ethyl-2-methylcyclohexane and (1S,2R)-1-ethyl-2-methylcyclohexane results. One compound has R,S configuration and the other has S,R configuration. By using the CIP rules priorities are given to the groups attached to the chiral carbon. CIP rules are based on the atomic masses of the groups attached so the minimum number is given to the group having the highest atomic mass. As it is a mixture of two enantiomers so we can call it a recemic mixture.
You can also learn about catalytic reduction from the following question:
https://brainly.com/question/14785855
#SPJ4
Describe the composition of calcite
Answers
Answer:
CaCO3
Explanation:
Pure calcite has the composition CaCO3. However, the calcite in limestone often contains a few percent of magnesium. Calcite in limestone is divided into low-magnesium and high-magnesium calcite, with the dividing line placed at a composition of 4% magnesium. High-magnesium calcite retains the calcite mineral structure, which is distinct from that of dolomite, MgCa(CO3)2.[18] Calcite can also contain small quantities of iron and manganese.[19] Manganese may be responsible for the fluorescence of impure calcite, as may traces of organic compounds