What is the percent composition of sodium in Na3PO4? Express your answer to one decimal place. No units required.
Answers
Answer:
42.07%
Explanation:
Molar mass of Na3PO4 = 23×3 + 31 +16×4
= 69+ 31+64= 164 g/mol
Mass of Na in this compound is 69
Percentage of sodium in sodium phosphate = 69/ 164×100
Percentage of Na = 42.07%
Related Questions
As the wavelength of an electromagnetic wave gets longer, the frequency ____________________.
Answers
Or
Decreases
Yoshi divided the mass of a rubber stopper by its volume. Using this calculation, Yoshi found the
of the stopper
A
force
B
density
С.
weight
D
buoyancy
Answers
Density = mass/volume because it’s basically the concentration of mass. How much matter takes up the space. If you pack more matter into less space, it is more dense.
Question 11 (1 point)
Scientists use comparative embryology to look into evolutionary relationships between animals, specifically those that look very
different from each other when they are fully formed.
True
False
Review Answers
Saved at 9:10 am
+ → X
Answers
HELP PLEASE I BEG ILL GIVE U 10 POINTS

Answers
which is more concentrated 200 ML of an 8-M NaOH solution or 500 mL of a 4-M NaOH solution
Answers
Answer:
200 ML of an 8-M NaOH solution; The higher the molarity, the higher the concentration of the substance. The volume of the 8M NaOH solution is less as well.
Molarity of a solution defines the concentration of a solution. It is the amount of the solute in 1 L of water. Mathematically it is expressed as:
\(Molarity = \frac{Mole}{Volume}\)
From the formula above, the molarity and the volume are in inverse proportionality.
Thus, an increase in the volume of water will leads to a decrease in the concentration while a decrease in the volume of water will lead to an increase in the concentration.
We shall compare both solutions as follow:
Solution 1:Volume = 200 mL
Molarity = 8 M
Solution 2:Volume = 500 mL
Molarity = 4 M
We can see that solution 1 has a lower volume than solution 2. Hence, the concentration of solution 1 is more than that of solution 2.
From the illustrations above, we can conclude that 200 mL of a 8M NaOH solution is more concentrated than 500 mL of a 4M NaOH solution.
Learn more: https://brainly.com/question/16587536
Which of the following people believed the atom to be indivisible?
Democritus
Dalton
Bohr
Rutherford
Answers
Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
What is an atom?Atom is a visible particle consist of proton neutron and electrons and can be seen under the electron microscope only and it is the smallest unit of matter from which everything of the universe made up of.
Dalton says that atom is the particle cannot be seen but all matter is made up of that only which is not true and not even possible because matter is visible to us.
After some time with the help of experiments Rutherford performed bombarding experiment on the gold foil with the help of that he concluded that atom is visible.
Therefore, Dalton believed that atom in not visible it is an invisible particle and whole universe is made up of atom only.
Learn more about Atom, here:
https://brainly.com/question/14214017
#SPJ5
5. Which of the following are examples of precipitation reactions? Select all that apply.
OKCl(ag) + AgNO3(aq) → KNO3(aq) + AgCl(s)
OHCl(aq) + NaOH(ag) → H₂O(1) + NaCl(aq)
O2 H2(g) + O2(g) → 2 H₂0 (1)
Mg(s) + O2(g) → MgO2 (s)
Answers
\(KCl(ag) + AgNO_3(aq)\) → \(KNO_3(aq) + AgCl(s)\) is the precipitation reactions.
What are precipitation reactions?A precipitate is an insoluble substance. A reaction in which insoluble solid precipitate is formed is called Precipitation Reaction.
For example, When Sodium Sulphate solution is mixed with Barium Chloride solution It forms Barium Sulphate and Sodium Chloride solution.
Precipitation reactions are usually double displacement reactions involving the production of a solid form residue called the precipitate.
These reactions also occur when two or more solutions with different salts are combined, resulting in the formation of insoluble salts that precipitate out of the solution.
Learn more about the precipitation reactions here:
https://brainly.com/question/24846690
#SPJ1
How many carbon atoms are there in a 12.5 kg sample of carbon?
step by step solution
Answers
Answer:
6.27 X 10^26 atoms
Explanation:
Write down the formula:
number of moles = mass/Ar (Ar is atomic mass)
Ar of carbon = 12
mass = 12.5kg or 12500g
Substitue these values into the formula:
number of moles = 12,500g / 12 = 3125/3 or 1041.6
Convert this to atoms:
1 mole = 6.022 X 10^23 atoms
So, 1041.6 moles = 6.27 X 10^26 atoms
What is an independent variable? and What is a dependent variable?
Answers
A dependant variable is the variable that depends on other factors that are measured
Answer:
An independent variable is defines as the variable that is changed or controlled in a scientific experiment. A dependent variable is the variable being tested in a scientific experiment. The dependent variable is "dependent" on the independent variable.
Explanation: x is the independent variable and y is the dependent variable
imagine you are frosting a cake apply pascal's law to using the bag of frosting what would happen when you squeeze the bag
Answers
What property of a metal does the image represent
Answers
Answer:
malleable
Explanation:
The image represent in malleable property of metal.
The image possibly represents the photoelectric effect of a metal, which is when it emits electrons after being exposed to electromagnetic radiation. Metals are also characterized by physical properties such as conductivity, malleability, metallic luster, and metallic bonding.
Explanation:Based on your question, the image possibly represents the photoelectric effect, a key property of metals. This phenomenon occurs when a metal surface exposed to electromagnetic waves of a certain frequency absorbs radiation and emits electrons. These emitted electrons are called photoelectrons. Metals can also exhibit free electron model behavior, where electrons freely roam within the metal structure.
Metals possess unique physical properties like conductivity, malleability, and metallic luster. Malleability refers to the metal's ability to deform without breaking, while conductivity refers to the metal's ability to transfer heat or electricity. A metallic luster gives metals their characteristic shiny appearance.
Finally, metals are also known for their metallic bonding—a unique force that holds together the atoms within a metallic solid. Metallic bonding gives rise to many useful and varied bulk properties of metals.
Learn more about Properties of Metals here:https://brainly.com/question/33514448
#SPJ2
Rank the following ions in order of decreasing size: Sr2+, Ba2+, Cs+. Also, please explain why.
I know that Sr2+ is smaller than Ba2+ because ionic radius increases down a group, but I do not know where Cs+ fits in.
Answers
The ranking of the ions in decreasing order of size is: Cs+ > Sr2+ > Ba2+.
The size of an ion is determined by its effective nuclear charge and the number of electron shells. Generally, as you move down a group in the periodic table, the number of electron shells increases, leading to an increase in the size of the ions.
In this case, we are comparing the sizes of Sr2+, Ba2+, and Cs+ ions.
Cs+ has the largest size among the three ions. Cs+ is an alkali metal ion, and as you move down Group 1 (alkali metals) in the periodic table, the size of the ions increases due to the addition of electron shells. Cs+ has one more electron shell compared to Sr2+ and Ba2+, making it the largest ion.
Next, we have Sr2+. Sr2+ is an alkaline earth metal ion and is smaller than Cs+ because it is located one row above Cs+ in the periodic table. Although both Sr2+ and Cs+ have the same number of valence electrons (one), Sr2+ has one less electron shell compared to Cs+, resulting in a smaller size.
Lastly, we have Ba2+. Ba2+ is also an alkaline earth metal ion but is larger than Sr2+. Ba2+ is located one row below Sr2+ in the periodic table, which means it has one additional electron shell. The additional electron shell increases the size of the Ba2+ ion compared to Sr2+.
Therefore, the ranking of the ions in decreasing order of size is: Cs+ > Sr2+ > Ba2+.
Learn more about Valence electrons from the link given below.
https://brainly.com/question/31264554
#SPJ4
how do i do this? it doesn’t make sense

Answers
Phosphate is any salt or ester of phosphoric acid with the chemical formula PO₄³-
According to this question, a chemical compound with the formula; FePO₄ was given. The iron ion has a 3+ charge and the phosphate ion has a 3- charge.
Charge of an ion can be estimated by finding the difference between the number of protons and electrons in the atom.
Phosphate is an ion with charge of -3, hence, it complements an iron ion with charge +3 to form a neutral compound.
Learn more about phosphate at: https://brainly.com/question/30594957
#SPJ1
according to the second law of thermodynamics, for any process that may occur within an isolated system, which one of the choices applies
Answers
According to the second law of thermodynamics, for any process that may occur within an isolated system, the total entropy of an isolated system can never decrease. This principle is often referred to as the entropy principle. What is the second law of thermodynamics? The second law of thermodynamics establishes the basic properties of the entropy of an isolated system, as well as the implications of those properties.
The total entropy of an isolated system can never decrease in a natural process. In other words, the total entropy of a system can never be less than its initial entropy. When a system reaches thermal equilibrium, the total entropy of the system is maximized. This is the most disorderly and disordered state of the system.
Why entropy is a fundamental property of the second law of thermodynamics? Entropy is a fundamental property of the second law of thermodynamics because it is a measure of the level of disorder in a system.
When a system reaches thermal equilibrium, it has the highest degree of disorder and entropy. According to the second law of thermodynamics, this condition is the most likely state of a system, and it is called the state of maximum entropy. Therefore, the second law of thermodynamics describes how the entropy of a system changes over time and how it affects the efficiency of energy conversion processes in the system.
Learn more about thermodynamics at https://brainly.com/question/31645316
#SPJ11
The mixture and elements of Coffee w Cream and Sugar
Answers
Is carbon Dioxide matter????
Answers
Answer:Yes
Explanation: Matter is everything around you.
How many molecules of oxygen are there in 1.00 dm3 of oxygen at standard pressure and a temperature of 273 K. Give your answer to three significant digits.
Answers
we need no of moles
\(\\ \tt\hookrightarrow PV=nRT\)
\(\\ \tt\hookrightarrow n=\dfrac{PV}{RT}\)
\(\\ \tt\hookrightarrow n=\dfrac{1}{273(8.314)}\)
\(\\ \tt\hookrightarrow n=1/2269.7=0.0004mol\)
No of molecules=No of moles×Avagadro no
\(\\ \tt\hookrightarrow 0.0004(6.023\times 10^{23})\)
\(\\ \tt\hookrightarrow 2.4\times 10^{20} molecules \)
Hi there!
Note: 1 mole of a any gas at ST and Pressure (273 K and 1 atm) occupies a volume of 22.4 dm^3
=> x mole of oxygen occupies 1.00 dm^3
Therefore, the mole of oxygen on 1.00 dm^3 is :
\(mole \: of \: oxygen = \frac{1.00 {dm}^{3} \times 1 \: mole}{22.4 \: dm^{3} } = 0.044642857 \: mole\)
Also note that Avogadros Constant says: 1 mole of any gas contains \(6.022 \times {10}^{23}\)molecules.
Hence, 0.044642857 mole of oxygen will contain :
\(0.044642857 \times 6.022 \times {10}^{23} = 2.69 \times {10}^{22} \: molecules\)
Therefore, \(2.69 \times {10}^{22} \: molecules \: are \: present \: in \: 1dm^{3} \: of \: oxygen \: at \: stp\)
I need help with this pleaseYou are cooking a dinner and the recipe calls for chicken broth. You realize that you don’t have a can of liquid broth, but you have the dried cube form of chicken broth that can be dissolved in water.

Answers
Answer
crush the cubes of broth, add warm water and stir the container.
Explanation
The FASTEST way to make the chicken broth with the cubes you have will be to increase the surface area of the cubes broth by crushing and raise the temperature of the cubes broth by adding warm water and by stirring the container.
Hence, the correct answer to your question is:
crush the cubes of broth, add warm water and stir the container.
Return all unused chemicals to their original containers true or false
Answers
Answer:
no
Explanation:Unused chemicals are never returned to their original containers because you will be contaminating the chemical. Dispose of unused chemicals in the proper containers.
What is the ATOMIC NUMBER for an element with 5 protons, 6 neutrons and 2 electrons?
Answers
The element's Atomic number , which has 5 protons, 6 neutrons, and 2 electrons (the atomic number is the number of protons) = 5
What in an atomic number?the number assigned to a chemical element according to its atomic number in the periodic system, which places the elements in ascending order of the number of protons in their nuclei. As a result, the atomic number is also the number of protons, which in a neutral atom is always equal to the amount of electrons.
Briefing:Protons and neutrons are added to determine an element's mass number. The only number that may change while maintaining the identity of an element is the number of neutrons for that element (because the atomic number is the number of protons).
Mass number = protons + neutrons
According to the given data;Given:
5 protons,
6 neutrons
2 electrons
Atomic number = ?
We know that the Mass number = protons + neutrons:
Mass number = protons + neutrons
Mass number = 5 + 6:
Mass number = 11
The element's Atomic number , which has 5 protons, 6 neutrons, and 2 electrons (the atomic number is the number of protons) = 5.
To know more about Atomic number visit:
https://brainly.com/question/16858932
#SPJ10
Which designation does NOT correlate molecule's mass with its correct associated units?
-Mr; Da
-m; kDa
-u; g
-m; MDa
Answers
The designation "Mr" does not correlate a molecule's mass with its correct associated units, as it is a relative measure of molecular mass and not expressed in a specific unit like Da, kDa, or g. Thus, Option A is correct.
"Mr" stands for relative molecular mass and is a dimensionless value that represents the mass of a molecule relative to the mass of a carbon-12 atom. In contrast, "Da" (Dalton) and "kDa" (kilodalton) are units of mass commonly used to express the molecular weight of a molecule, while "g" (gram) is a standard unit of mass. "MDa" (megadalton) is also a unit of mass, but it is a larger unit than "kDa."
Therefore, only "Mr; Da" does not provide a specific unit for the mass of a molecule and cannot be used to directly convert to other units.
Learn more about molecule's mass brainly.com/question/24191825
#SPJ11
what is a mixture of elements and compounds

Answers
The substance in the image above would be classified as a mixture of elements (option E).
What is a compound and mixture?A compound is a substance formed by chemical bonding of two or more elements in definite proportions by weight.
On the other hand, a mixture is made when two or more substances are combined, but they are not combined chemically.
According to this question, an image is shown with two different substances or elements as distinguished by coloration (white and purple). These elements are combined but not chemically bonded, hence, is a mixture.
Learn more about mixture at: https://brainly.com/question/12160179
#SPJ1
The beaker contains a 25% salt solution and the bag inside contains pure water. The bag is semi-permeable. A: Explain which direction the water will move. B: explain a scenario where water might move the other direction
Answers
The direction of the water between the beaker containing a 25% salt solution and the bag inside with pure water would be from beaker to bag with pure water.
If the beaker had pure water and the bag with salt solution, the water would move from bag to beaker with pure water.
The beaker has salt solution (salt (solute) + water (solvent)). The bag has pure water without any solute. The water normally moves from higher concentration to lower concentration.
Water containing solute would have higher concentration than pure water. Thus, water moves from beaker with higher concentration to bag with lower concentration. The process is called osmosis.
Similarly, if we change the components in beaker and bag, the water would move from bag with higher concentration to beaker with pure water of lower concentration.
To know more about Concentration
https://brainly.com/question/10720472
#SPJ1
help
whoever answers all gets marked brainliest

Answers
#1
See H and C have shared their electrons so it's Covalent bonding#2
Yes here we can see the dots and crosses clearly.
Dot and crosses digram#3
It's Methane or CH_4
4 single bonds#4
There is no double bond
Answer:
#1
See H and C have shared their electrons so its Covalent bonding
#2
Yes, here we can see the dots and crosses clearly.
Dot and crosses diagram
#3
It's Methane or CH_4
4 single bonds
#4
There is no double bond
Explanation:
mark the person that did it before me brainliest
Calculate the acid ionization constant (Ka) for the acid. Express your answer using two significant figures. IVO AO ? K. = Submit Request Answer A 0.120 M solution of a weak acid (HA) has a pH of 3.28. You may want to reference (Pages 737 - 745) section 16.6 while completing this problem.
Answers
Answer : The acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
To calculate the acid ionization constant (Ka) for the given acid HA, we must first find its pH using the given concentration of the solution. Then, we can use the pH to find the concentration of H+ ions in the solution. Finally, we can plug these values into the expression for Ka to solve for the acid ionization constant.
The pH of the 0.120 M solution of HA is given to be 3.28. This means that [H+] = 10^(-pH) = 10^(-3.28) = 5.01 x 10^(-4) M.
Now, we can use the expression for Ka: Ka = [H+][A-]/[HA], Since HA is a weak acid, we can assume that it dissociates as follows: HA + H2O ⇌ H3O+ + A- This means that [A-] = [H3O+], and [HA] = initial concentration of the acid (0.120 M) - [H3O+].
Substituting these values, we get: Ka = (5.01 x 10^(-4) M)^2 / (0.120 M - 5.01 x 10^(-4) M) = 1.1 x 10^(-5). Therefore, the acid ionization constant (Ka) for the given acid HA is 1.1 x 10^(-5), rounded to two significant figures.
Know more about acid ionization constant here:
https://brainly.com/question/4110687
#SPJ11
For the reaction H2(g) +S(g) – H2S(g) the initial concentrations are 0.0550 M H2,0.0800 M S, and no H2S. At equilibrium, [H2] = 0.0100 M.Part 1 (2 pts) Feedback See Periodic Table See Hint Calculate the concentrations of S and H2S at equilibrium. (Be sure to give your answers to three decimal places.) ___MS ____MH2S Part 2 (1 pt) Calculate the value of Kunder the reaction conditions at equilibrium.
Answers
Part 1: The concentrations of S and H2S at equilibrium are 0.0575 M and 0.000575K M, respectively.
Part 2: The value of K under the reaction conditions at equilibrium is 0.994 M^-1.
Part 1:
The balanced chemical equation for the reaction is:
H2(g) + S(g) ↔ H2S(g)
At equilibrium, the concentration of H2 is given as 0.0100 M. Let the concentrations of S and H2S at equilibrium be x and y, respectively. Then, the equilibrium expression can be written as:
K = [H2S]/[H2][S]
Substituting the given values, we get:
K = y/(0.0100)(x)
To calculate the concentrations of S and H2S, we need to use the equilibrium constant expression and the given equilibrium concentrations:
K = [H2S]/[H2][S]
0.0550 - 0.0100 = 0.0450 M H2 is consumed to reach equilibrium.
0.0800 - x = 0.0800 - (0.0450/2) = 0.0575 M S is consumed, and the equilibrium concentration of S is x = 0.0575 M.
The equilibrium concentration of H2S is y = K[H2][S] = (0.0100)(0.0575)K = 0.000575K.
Therefore, the concentrations of S and H2S at equilibrium are 0.0575 M and 0.000575K M, respectively.
Part 2:
The equilibrium constant expression is:
K = [H2S]/[H2][S]
Substituting the equilibrium concentrations and solving for K, we get:
K = [H2S]/[H2][S] = (0.000575K)/(0.0100)(0.0575) = 0.994 M^-1.
Therefore, the value of K under the reaction conditions at equilibrium is 0.994 M^-1.
For more such questions on Equilibrium concentrations.
https://brainly.com/question/15300000#
#SPJ11
can someone help me with this please

Answers
Answer:
25 ml
Explanation:
it will be 25 ml only
How can a reaction generally be made to go more quickly?Check all that apply.A.Cooling the reactionB.Adding a catalystC.Lowering the activation energyD.Shaking the reactants

Answers
Answer
A reaction generally can be made to go more quickly by:
B. Adding a catalyst
C. Lowering the activation energy
D. Shaking the reactants
Explanation
The presence of a catalyst increases the rate of a reaction by lowering its activation energy. Chemical reactions occur when molecules collide with each other and undergo a chemical transformation.
Stirring and shaking provide some kinetic energy to the system. It increases slightly its temperature and the reaction rate. increasing the kinetic energy of reactant particles also means more of the reactant particles will have the minimum amount of energy required to form products (ie, activation energy) which leads to more successful collisions and therefore increases the reaction rate.
Which substance would require the most energy to heat 10.00 g of it by 10.0°C?
O Ice, c = 2.03 Jigº
O Mercury, c = 0.140 J/gºC
O Water, c = 4.18 J/gºC
O Aluminum, c = 0.902 J/gºC
Answers
Answer:
a
Explanation:
a
please help when ever it lets me give brainliest i will give it
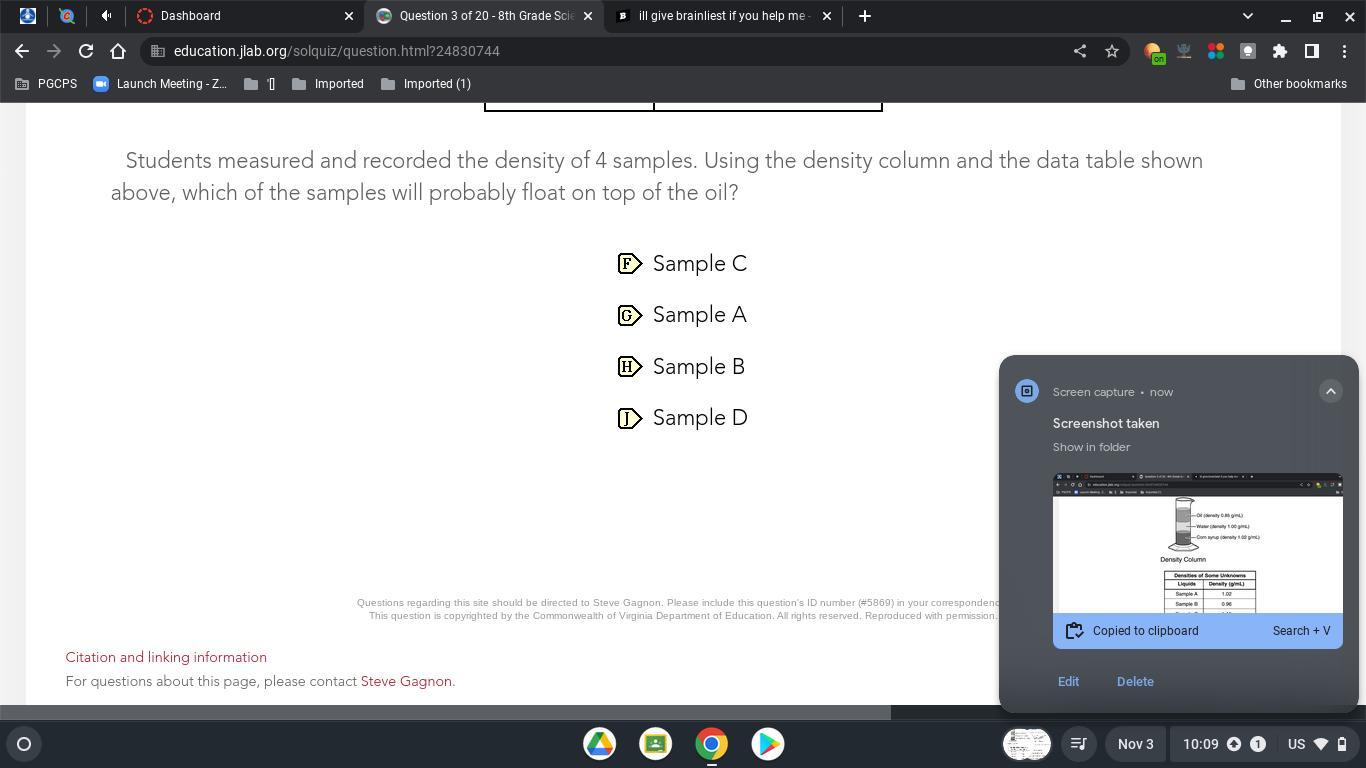

Answers
Answer:
it's sample c yh it is true