what is the solubility of cr(oh)₃ at a ph of 11.00? (ksp cr(oh)₃ is 6.70 × 10⁻³¹)
Answers
The solubility of Cr(OH)₃ at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L. at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L.
Given that the Ksp of Cr(OH)₃ is 6.70 × 10⁻³¹ at a pH of 11.00.To find the solubility of Cr(OH)₃, we use the expression for the solubility product. It is given as:Ksp = [Cr³⁺][OH⁻]³We know that at a pH of 11, the concentration of OH⁻ ions is 1.0 × 10⁻³ M.
The reaction is given as:Cr(OH)₃(s) ⇌ Cr³⁺(aq) + 3OH⁻(aq)The concentration of Cr³⁺ is the same as the solubility of Cr(OH)₃(s).Let the solubility of Cr(OH)₃ be x. Then, the concentration of Cr³⁺ is x.[OH⁻]³ = Ksp/Cr³⁺(1.0 × 10⁻³)³ = 6.70 × 10⁻³¹/xx = (6.70 × 10⁻³¹/(1.0 × 10⁻³)³)¹∕³= 4.69 × 10⁻¹⁰ mol/LTherefore, the solubility of Cr(OH)₃ at a pH of 11.00 is 4.69 × 10⁻¹⁰ mol/L.
To know more about solubility visit:
https://brainly.com/question/31493083
#SPJ11
Related Questions
HELP!! b. At the equivalence point, all of the acid has been neutralized by the base. Why does the pH change so
sharply around the equivalence point? (HINT: the pH is neutral, just like the water example in the
previous question.)
Answers
At equivalence there is no more HA and no more NaOH, for this particular reaction. So that means we have a beaker of NaA and H2O. The H2O contributes 1 x 10-7 M hydrogen ion and hydroxide ion. But NaA is completely soluble because group 1 ion compounds are always soluble. So NaA breaks apart in water and it just so happens to be in water. So now NaA is broken up. The Na+ doesn't change the pH but the A- does change the pH. Remember that the A anion is from a weak acid. That means it will easily attract a hydrogen ion if one is available. What do you know? The A anion is in a beaker of H+ ions! So the A- will attract H+ and become HA. When this happens, it leaves OH-, creating a basic solution, as shown below.
Question 1
How many moles of oxygen are produced when 18.02 moles of dihydrogen dioxide decompose?
2H₂O2 (1)--> O2 (g) + 2H₂O (1) (balanced)
Answers
The number of moles of oxygen is 9.01 moles
What is the use of mole in stoichiometry?The mole is a fundamental unit of measurement in chemistry and is used extensively in stoichiometry, which is the study of the quantitative relationships between reactants and products in a chemical reaction.
In stoichiometry, the mole is used to quantify the amount of a substance involved in a reaction. The mole is defined as the amount of a substance that contains the same number of particles (atoms, molecules, ions, or other entities) as there are in 12 grams of pure carbon-12.
We know that;
2 moles of H₂O2 produces 1 mole of oxygen
18.02 moles of H₂O2 produces 18.02 * 1/2
= 9.01 moles
Learn more about moles:https://brainly.com/question/26416088
#SPJ1
If a block of aluminum has a mass of 148 g and the water has a volume of 220 mL(density of water= 1g/mL). What will the final temperature of the substance be?
Answers
The final temperature of the substance cannot be determined without knowing the initial temperature or additional information.
What is the final temperature of the substance without knowing the initial temperature or additional information?To calculate the final temperature of the substance, we need to consider the heat exchange between the aluminum block and the water.
This can be done using the principle of conservation of energy.
First, let's determine the mass of water in grams, as the density of water is given as 1 g/mL and the volume of water is 220 mL:
Mass of water = Volume of water x Density of water
Mass of water = 220 mL x 1 g/mL
Mass of water = 220 g
Next, we need to determine the heat exchanged between the aluminum block and the water. The heat exchanged can be calculated using the formula:
q = m ˣ c ˣ ΔT
Where:
q is the heat exchanged
m is the mass of the substance (either aluminum or water)
c is the specific heat capacity of the substance
ΔT is the change in temperature
Assuming the specific heat capacity of aluminum is 0.897 J/g°C, and we have no information about the initial or final temperatures, we cannot calculate the final temperature of the substance accurately.
The specific heat capacity of water is approximately 4.18 J/g°C.
If you provide the initial temperature of the system or any additional information, I can assist you further in calculating the final temperature.
Learn more about initial temperature
brainly.com/question/2264209
#SPJ11
Why does less evaporation mean higher temperatures in urban areas?
Answers
Less evaporation means that less of the sun's energy is used to convert water into water vapor, and more of it is used to heat up the surface. In urban areas, there is typically less vegetation and more impervious surfaces (such as concrete and asphalt), which reduces the amount of water that can evaporate.
This means that more of the sun's energy is absorbed by the surface, leading to higher temperatures. Additionally, buildings and other structures in urban areas can trap heat and prevent it from dissipating, further contributing to the urban heat island effect.
In rural areas, vegetation and soil moisture play an important role in regulating temperature through a process called evapotranspiration. Evapotranspiration is the combined process of water evaporation from the soil and plant transpiration. It helps to cool the air by removing heat from the surface through the transfer of water from the surface to the atmosphere.
In contrast, urban areas have a significant amount of impervious surfaces like concrete, asphalt, and buildings, which reduce the amount of vegetation and soil moisture. As a result, urban areas have less evapotranspiration, which means less cooling effect from the evaporation of water. This leads to a higher surface temperature in urban areas.
Furthermore, urban areas have a higher proportion of dark-colored surfaces, such as asphalt and concrete, which absorb more solar radiation than lighter-colored surfaces like vegetation and soil. This is known as the "urban heat island effect," which further contributes to higher temperatures in urban areas.
For more question on evaporation click on
https://brainly.com/question/8944874
#SPJ11
What is the Ka of a 0.0981 M
solution of hydrocyanic acid
(HCN) with a pH of 6.00?
Ka = [?] x 10!?)
Answers
Answer:
Attached picture
Ka = [?] x 10
1.02 x 10^-11 = [?] x 10
[?] = 1.02 (I'm assuming you're asking this)
(If not then divide the 10)
![What is the Ka of a 0.0981 Msolution of hydrocyanic acid(HCN) with a pH of 6.00?Ka = [?] x 10!?)](https://i5t5.c14.e2-1.dev/h-images-qa/answers/attachments/ypLsqacfcjKjqW7XIR7CnFDKLookhsbd.jpeg)
Answer:
The answer is 1.02x10^-11
Explanation:
Fill in the box 1.02 and then -11
What causes day and night?
Make sure you give a full, detailed answer, not just one sentence.
Answers
Answer:
The earth's rotation, earth is rotating, so one side of earth will be hit by the sun, and the other wont be hit, making it dark, but since earth rotates, it causes it to turn, making one side have daylight, and the other, darkness, which the light you see is from the sun's reflection on the moon
Hope this helps
Can somebody help me I’ve been a helping hand all day and nobody helping like does the app work or I need to delete.
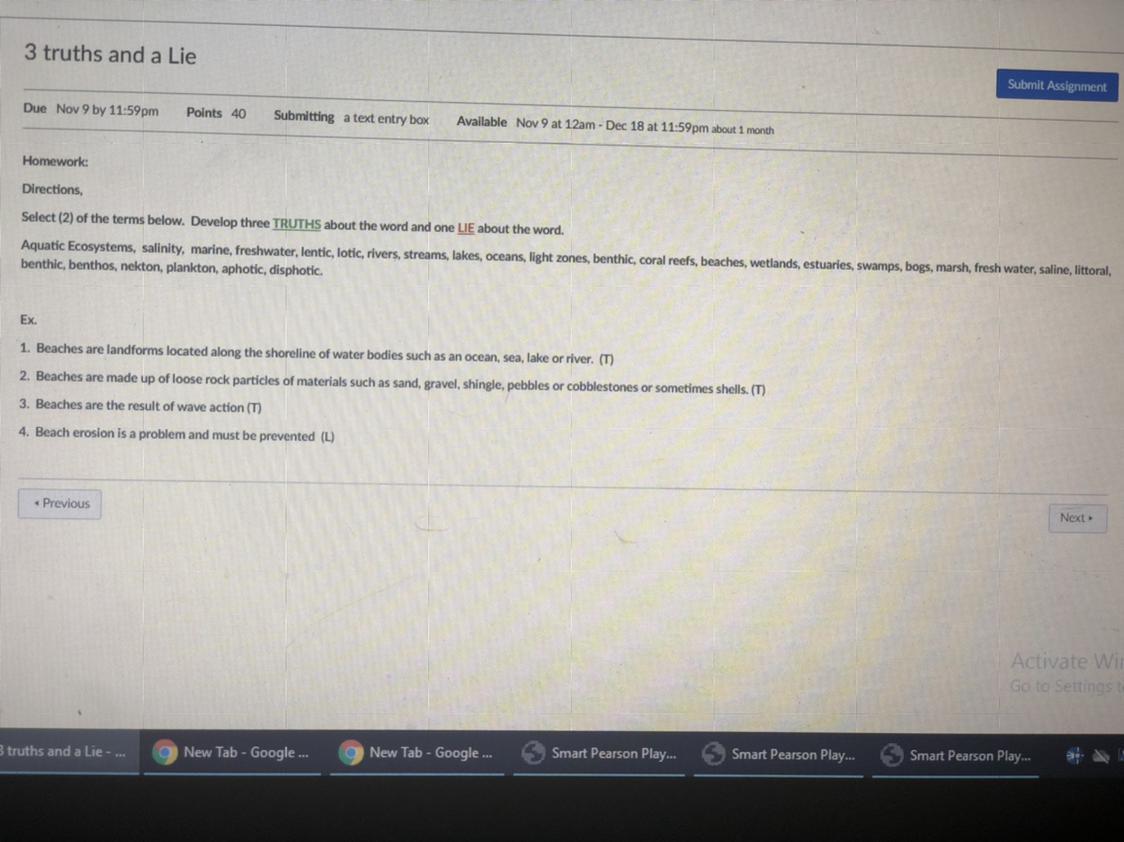
Answers
I believe that #1 is the lie, but I'm not great at this subject.
A car is moving with the velocity of 20m/s. After 5 seconds it's velocity becomes 50m/s. Find its acceleration.
Answers
Answer:
6 m/s^2
Explanation:
a= (v2 - v1)/t
= (50-20)/5
=6
Answer:
6 m/s²
Explanation:
Given, Initial velocity (u) = 20m/s
Final velocity (v) = 50m/s
time taken (t) = 5 sec
Now,
Acceleration = v-u/t
= 50-20/5
= 30/5
= 6 m/s²
one cheeseburger has 340 kcal and 25 grams of fat. what percentage of total kcal comes from fat? (round to the nearest whole number)
Answers
Percentage of total kcal ( total calories ) comes from fat is 66%
To determine the percentage of total calories that come from fat in a cheeseburger, we first need to calculate the number of calories from fat. Fat has 9 calories per gram, so 25 grams of fat would provide 25 x 9 = 225 calories.
Next, we divide the number of calories from fat by the total number of calories in the cheeseburger and multiply by 100 to find the percentage:
(225 / 340) * 100% = 66.18%
Rounding to the nearest whole number, we can say that 66% of the total calories in a cheeseburger come from fat.
Learn more about total calories here
https://brainly.com/question/1357152
#SPJ4
I need help asap on balancing equations

Answers
The given chemical equation is:
MgCl + Na(OH)₃ → Mg₃(OH)₂ + NaCl
To balance this equation, we need to ensure that the number of atoms of each element is the same on both sides of the equation.Let's start by balancing the magnesium (Mg) atoms:MgCl + Na(OH)₃ → Mg₃(OH)₂ + NaCl
There are three magnesium atoms on the right side (Mg₃) but only one on the left side. To balance the magnesium, we need to place a coefficient of 3 in front of MgCl:3 MgCl + Na(OH)₃ → Mg₃(OH)₂ + NaCl
Next, let's balance the chlorine (Cl) atoms:There are three chlorine atoms on the left side (3 Cl) but only one on the right side. To balance the chlorine, we need to place a coefficient of 3 in front of NaCl:3 MgCl + Na(OH)₃ → Mg₃(OH)₂ + 3 NaCl
Now let's balance the sodium (Na) atoms:There is one sodium atom on the left side but three sodium atoms on the right side. To balance the sodium, we need to place a coefficient of 3 in front of NaOH:3 MgCl + 3 Na(OH)₃ → Mg₃(OH)₂ + 3 NaCl
Finally, let's balance the hydrogen (H) and oxygen (O) atoms:There are nine hydrogen atoms on the right side (6 in Mg₃(OH)₂ and 3 in 3 (NaOH), but only three on the left side. To balance the hydrogen, we need to place a coefficient of 6 in front of H₂O:3 MgCl + 3 Na(OH)₃ → Mg₃(OH)₂ + 3 NaCl + 6 H₂O
There are six oxygen atoms on the right side (2 in Mg₃(OH)₂ and 6 in 3 NaOH), but only three on the left side. To balance the oxygen, we need to place a coefficient of 3 in front of Mg(OH)₂:3 MgCl + 3 Na(OH)₃ → 3 Mg₃(OH)₂ + 3 NaCl + 6 H₂O
Now the equation is balanced with the same number of atoms on both sides.For such more questions on Balancing equations
https://brainly.com/question/26694427
#SPJ8
If 1 atom of sulfur combines with 2 atoms of oxygen to form sulfur dioxide, what is the ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide?
Answers
Answer: The ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.
Explanation:
Law of constant proportion states that In a chemical substance the elements are always present in definite proportions by mass. This law is also known as 'Law of definite proportions '.
Mass of 1 atom of sulphur = 32 g
Mass of 1 atom of oxygen = 16 g
Mass of 2 atoms of oxygen =\(16g\times 2=32g\)
In formation of \(SO_2\) , 1 atom of sulfur combines with 2 atoms of oxygen and thus the mass ratio will be 32: 32= 1:1 .
Thus the ratio of mass of sulfur atom to mass of oxygen atom in sulfur dioxide is 1: 1.
Which of the following statements is one
of the postulates in Dalton's atomic
theory?
a. All elements are composed of atoms.
b. Most elements are composed of atoms.
c. Some elements are composed of atoms.
d. Solid elements are composed of atoms.
Answers
Answer:
a. All elements are composed of atoms.
Explanation:
dalton's atomic theory says that Compounds are composed of atoms of more than 1 element. The relative number of atoms of each element in a given compound is always the same. Chemical reactions only involve the rearrangement of atoms. Atoms are not created or destroyed during chemical reactions.
125 J of energy are released when 25.0 g Si comes into contact with cold water. What is the change in temperature for the silicon?
Note: q = -125 J
Answers
The change in temperature for the silicon is approximately -2.21°C. Note that the negative sign indicates a decrease in temperature.
What is the specific heat capacity of silicon if its temperature decreases by 5.0 degrees Celsius when 125 J of energy are released?Using the formula q = m * c * ΔT, we can rearrange it to solve for c, giving c = q / (m * ΔT). Plugging in the given values, we get c = -125 J / (25.0 g * -5.0 degrees Celsius) = 1.00 J/(g*C).
To calculate the change in temperature of silicon, we need to use the specific heat capacity of silicon (0.71 J/g°C) and the equation:
q = m × c × ΔT
where q is the energy released (-125 J), m is the mass of silicon (25.0 g), c is the specific heat capacity of silicon (0.71 J/g°C), and ΔT is the change in temperature we want to calculate.
Rearranging the equation, we get:
ΔT = q / (m × c)
Substituting the values, we get:
ΔT = -125 J / (25.0 g × 0.71 J/g°C) ≈ -2.21°C
Learn more about silicon here:
https://brainly.com/question/28213172
#SPJ1
Answer:-29.4
Explanation:
What is the concentration of a solution made by combining 52 grams of NaCH3COO with 150 mL of water?
Answers
Answer: The concentration of a solution made by combining 52 grams of \(NaCH_{3}COO^{-}\) with 150 mL of water is 4.22 M.
Explanation:
Given: Mass = 52 grams
Volume = 150 mL (1 mL = 0.001 L) = 0.150 L
Molarity is the number of moles of a substance present in liter of a solution.
And, moles is the mass of a substance divided by its molar mass.
Hence, moles of \(NaCH_{3}COO^{-}\) (molar mass = 82.034 g/mol) is as follows.
\(Moles = \frac{mass}{molar mass}\\= \frac{52 g}{82.034 g/mol}\\= 0.633 mol\)
Therefore, concentration of given solution is as follows.
\(Molarity = \frac{moles}{Volume(in L)}\\= \frac{0.633 mol}{0.150 L}\\= 4.22 M\)
Thus, we can conclude that the concentration of a solution made by combining 52 grams of \(NaCH_{3}COO^{-}\) with 150 mL of water is 4.22 M.
Do you think it's possible for a renewable resource such as water to become depleted or
reach near depletion? Explain.
Answers
define the following terms
A) chemical family
B) period
C) metalloid
Answers
Answer:
Chemical Family means a group of elements in the Periodic Table or, more commonly, compounds that share certain physical and chemical characteristics and have a common name.
A period in the periodic table is a row of chemical elements.
metalloid is an element (e.g. arsenic, antimony, or tin) whose properties are intermediate between those of metals and solid nonmetals or semiconductors.
How many grams of iron(III) sulfate, Fe2(SO4)3, are produced in the reaction if 2. 25 moles of hydrogen gas are produced? (round two decimal places)
Answers
The mass of iron(III) sulfate comes out to be 899.73 g, the calculations are shown below.
Considering, the moles of Fe₂(SO₄)₃ to be 2.25 moles.
Molar mass of Fe₂(SO₄)₃ = 399.88 g/mol.
To calculate the number of moles, the below formula is used-
Number of moles = Mass/molar mass
Substituting the known values in the above equation as follows-
2.25 moles = Mass / 399.88 g/mol
Mass = 2.25 moles x 399.88 g/mol
= 899.73 g
Therefore, the mass of iron(III) sulfate comes out to be 899.73 g.
To learn more about molar mass check the link below-
https://brainly.com/question/837939
#SPJ4
compare pure substance with mixture
Answers
Answer:
here
Explanation:
a pure substance consists only of one element or one compound. a mixture consists of two or more different substances, not chemically joined together.
A 10.0 gram sample of Fe contains how many miles of Fe
Answers
Answer:
1.7857 moles
Explanation:
moles=mass/Mr
10/56=1.7857
moles of iron =1.7857

bằng phương pháp hóa học hãy nhận biết các chất khí không màu trong 4 lọ không nhãn gồm:CO,O2,H2,CO2
Answers
Answer:
What??
Explanation:
I really do want to answer you but i dont understand your letters
waht is that??????
Which action would increase the reaction rate of a chemical reaction in aqueous solution?
O adding excess cold water
O cooling the reaction mixture
O increasing the surface area of reactants
O removing a catalyst
Answers
sumita m, sakata k, hayakawa y, asai s, miyasaka k, tanemura m. double percolation effect on the electrical conductivity of conductive particles filled polymer blends. colloid polym sci 1992;270(2):134
Answers
The article titled "Double Percolation Effect on the Electrical Conductivity of Conductive Particles Filled Polymer Blends" by Sumita M, Sakata K, Hayakawa Y, Asai S, Miyasaka K, and Tanemura M, was published in the journal Colloid and Polymer Science in 1992.
The article discusses the phenomenon of double percolation effect on the electrical conductivity of conductive particle-filled polymer blends. Percolation refers to the formation of interconnected pathways within a material, allowing for the flow of electricity.
In this study, the authors investigate how the electrical conductivity of polymer blends changes when conductive particles are added. They observe that two percolation thresholds exist: one for the polymer matrix and another for the conductive particles. The double percolation effect occurs when both thresholds are crossed, resulting in a significant increase in electrical conductivity.
Understanding the double percolation effect is important for designing materials with desired electrical properties. By controlling the composition and distribution of conductive particles within the polymer matrix, one can optimize the electrical conductivity of the blend.
To know more about Percolation Effect visit:-
https://brainly.com/question/30410369
#SPJ11
What volume i occupied by 22. 0 g of methane (CH4) at 27 degree Celiu and 1. 59 atm?
Answers
The volume occupied by 21.0 g of methane (CH4) at 27 degree Celsius and 59 atm is 26.1403 L.
What is meant by ideal gas law?A hypothetical gas made up of molecules that abide by the following conditions is referred to as an ideal gas: Molecular interactions in ideal gases are neutral. The molecules of an ideal gas wouldn't interact with each other or the container walls other than when they collided in an elastic collision.
The ideal gas law equation can be used to determine the volume occupied by a gas and is as follows:
PV = nRT
Where;
P = pressure (atm)
V = volume (L)
n = number of moles (mol)
R = gas law constant (0.0821 L atm/mol K)
According to this question;
n = 27g ÷ 16g/mol = 1.6875 mol
V = ?
P = 59 atm
T = 27°C + 273 = 300K
substitute the value sin the above equation, we get
1.59 × V = 1.6875 × 0.0821 × 300
simplifying the above equation, we get
1.59 V = 1.6875 × 0.0821 × 300
V = 26.1403 L
Therefore, volume occupied by 21.0 g of methane (CH4) at 27 degree Celsius and 59 atm is 26.1403 L.
To learn more about ideal gas law refer to:
brainly.com/question/4147359
#SPJ4
am i doing this right??? if not how can i change it?!

Answers
Answer:
yes you are doing it right my chemistry teacher said said so
Why is the vapor pressure of a warm lake higher than the vapor pressure of a cold lake?
Answers
Answer:
Explanation:
Warm lake vapor pressure is higher than cold lake vapor pressure because it evaporates more quickly. In other words, as the temperature of warm water increases, the kinetic energy of the warm water molecule also increases. As the kinetic energy of the warm water molecule increases, the number of molecules conversion into a vapor also increases, thereby increasing the vapor pressure of warm water.
Answer:
B.
Warm water evaporates more quickly.
Explanation:
i got it right on edmentum!!
molecule x contains a sugar and a phosphate group. what is molecule x ?
Answers
Find the volume of a liquid if 32.5 g of the liquid has a density of 0.852 g/mL.
Answers
Answer: 27.69 mL^3
Explanation:
32.5 g * 0.852 g/mL=
32.5*0.852=27.69 mL^3 ==>Volume is expressed in units cubed.
in chromatography, components of a mixture spend some time absorbed in a stationary phase and some time dissolved in a mobile phase. in terms of intermolecular forces and polarity of the molecules, explain how the components can be separated with these two phases
Answers
The two separate "phases" that make up chromatography are the mobile phase, which is the solvent that passes through the paper transporting various compounds, and the stationary phase. The stationary phase does not pass through the paper; it is contained there.
What is a chromatography?Chromatography is a technique for separating the components of a mixture. To begin the process, the mixture is dissolved in a substance known as the mobile phase, which then carries it through a substance known as the stationary phase. Chemical analysis uses chromatography, a laboratory technique, to separate a mixture into its component parts. The stationary phase is a material that is fixed in a system, and the mobile phase, which is a liquid solvent, dissolves the mixture and carries it through that system.
Why is chromatography important?Chromatography is the most used separation process in chemical laboratories where it is used for analysis, isolation, and purification. It is frequently used in both small- and large-scale production in the chemical process sector.
To know more Chromatography visit:
https://brainly.com/question/29485560
#SPJ4
If you leave your home at noon, and the speed limit is 50 km/h on the highway, what is the approximate time will you get to Atlantic City if Atlantic City is 150 km away? (obeying the speed limit of course)
Answers
Answer:
The time you will get to Atlantic City is 3 pm
Explanation:
Given;
speed limit, v = 50 km/h
distance to be traveled, d = 150km
The time of the motion is given as;
time = distance / speed
t = (150 km) / (50 km/h)
t = 3 hours
Thus, if you leave your home at noon, the time you will get to Atlantic City is given as;
time to get home = 12 pm + 3hours = 3 pm
Therefore, the time you will get to Atlantic City is 3 pm
What is the total number of electrons in the sixth energy level (n = 6).?
Answers
The total number of electrons in an energy level (n) can be determined using the formula:
Total number of electrons = 2n²
An electron is a subatomic particle that carries a negative electric charge. It is one of the fundamental particles that make up atoms. Electrons are located outside the atomic nucleus in specific energy levels or orbitals. They are incredibly small and have a mass of approximately 9.11 x 10^-31 kilograms.
For the sixth energy level (n = 6), we can substitute the value into the formula:
Total number of electrons = 2(6)²
= 2(36)
= 72
Therefore, the total number of electrons in the sixth energy level is 72.
For more details regarding electrons, visit:
https://brainly.com/question/12001116
#SPJ1