what mass of hcl would be needed to produce 9.8 moles of water?
Answers
Answer:
16 g
Explanation:
Hope This Helps
Happy Hoildays
~Zero~
Answer: i6g
Explanation:The important thing to notice here is that the reaction takes place at STP conditions, which are defined as a pressure of
100 kPa
and a temperature of 0∘C.
Related Questions
How many liters are in 4.5 moles of CO2 gas at STP? Pls help :)
Answers
Answer:
Explanation:
NCO 2= 4,5
VCO2= 4,5* 22,4=100,8
You examined the effects of heat on equilibrium in part iv. Was the reaction you observed endo- or exo- thermic? does heat act as a reactant or product? what happened when you removed heat by placing the solution in the ice bath? what would you expect to happen if you were to add heat?.
Answers
1) The reaction is exothermic and heat is a product
2) The color would turn to pink.
What is an endothermic reaction?Let us recall that an endothermic reaction is one in which the increase in the temperature of the system would drive the forward reaction. This implies that the equilibrium would shift to the right when the set up is heated. Since the color of the solution changes to blue when heated hence the reaction is exothermic.
If you removed heat by placing the solution in the ice bath, we can see that we have removed the heat thus we expect that the reverse reaction would take place and the color would change to pink.
Learn ore about exothermic reaction:https://brainly.com/question/10373907
#SPJ1

name an element in the fourth period (row) of the periodic table with: a. five valence electrons b. four 4p electrons c. three 3d electrons d. a complete outer shell
Answers
The metals of fourth period are Arsenic, Germanium, Scandium and Xenon.
(a)
Fourth Period
Five valence electrons
Five valence electrons means: This is V A group element.
V A group elements are: N, P, As, Sb, Bi
N = 7: 1s² 2s² 2p³
P = valence configuration = 3s²3p³
As valence configuration = 4s²4p³
So, the metal is As (Arsenic).
(b)
Fourth Period
Two 4-p electrons
Four valence electrons means: This is IV A group element.
IV A group elements are: C, Si, Ge, Sn, Pb
C = 6: 1s² 2s² 2p²
Si = valence configuration = 3s²3p²
Ge valence configuration = 4s²4p²
So, the metal is Ge (Germanium).
(c)
Fourth Period + one 3d electron
It is d-block element.
Sc = 21: 1s² 2s² 2p⁶3s²3p⁶4s²3d¹
3d¹ (one 3d one electron)
4s² (indicates fourth period)
Hence, the metal is Sc (scandium).
(d)
Fifth period + complete outer-shell
Complete outer-shell elements = He, Ne, Ar, Kr, Xe, Rn
He = 2: 1s²
Ne = 10: 1s² 2s² 2p⁶
Ar outer-shell = 3s²3p⁶
Xe outer-shell = 5s²5p⁶
Hence the metal is Xenon (Xe).
Learn more about elements from the link given below:
https://brainly.com/question/9410546
#SPJ4
you have a 12 ml sample of acetylcholine (a neurotransmitter) with an unknown concentration and a ph of 7.63. you incubate this sample with the enzyme acetylcholinesterase to convert all of the acetylcholine to choline and acetic acid. the acetic acid dissociates to yield acetate and hydrogen ions. at the end of the incubation period, you measure the ph again and find that it has decreased to 6.78. assuming there was no buffer in the assay mixture, determine the number of nanomoles of acetylcholine in the original 12 ml sample.
Answers
The number of nanomoles of acetylcholine in the original 12 ml sample is 2676.6.
Based on the given information, we can assume that the decrease in pH is due to the dissociation of acetic acid into acetate and hydrogen ions. Since there was no buffer in the assay mixture, the pH change indicates that the sample is not able to resist changes in acidity.
To determine the number of nanomoles of acetylcholine in the original 12 ml sample, we can use the following steps:
1. Calculate the change in hydrogen ion concentration:
Δ[H⁺] = 10^(pH₂ - pH₁) = 10^(6.78 - 7.63) = 0.223
2. Since acetic acid dissociates to yield one hydrogen ion, we can calculate the concentration of acetic acid in the sample:
[HAc] = Δ[H⁺] = 0.223 M
3. We can then use the balanced equation for the conversion of acetylcholine to choline and acetic acid:
Acetylcholine + H₂O → Choline + Acetic acid
Since all the acetylcholine is converted to acetic acid, we can assume that the initial concentration of acetylcholine is equal to the final concentration of acetic acid:
[Acetylcholine] = [HAc] = 0.223 M
4. To convert the concentration to nanomoles, we can use the formula:
n = C x V x 10^9
where n is the number of nanomoles, C is the concentration in M, and V is the volume in liters.
Since the volume of the sample is 12 ml or 0.012 L, we can calculate the number of nanomoles of acetylcholine in the sample:
n = 0.223 x 0.012 x 10^9 = 2676.6 nanomoles
Therefore, there were 2676.6 nanomoles of acetylcholine in the original 12 ml sample.
Learn more about acetylcholine at https://brainly.com/question/27960161
#SPJ11
the reaction of methyl iodide with sodium azide, nan3, proceeds by an sn2 mechanism. what is the effect of doubling the concentration of nan3 on the rate of the reaction? a. the rate remains the same b. the rate decreases by a factor of 2 c. the rate increases by a factor of 2 d. the rate increases by a factor of 4
Answers
The reaction of methyl iodide with sodium azide, NaN₃, proceeds by an SN2 mechanism. The effect of doubling the concentration of NaN₃ on the rate of the reaction is the rate increases by a factor of 2. The correct answer is C.
The alkyl halide is attacked by the nucleophile as the SN2 mechanism. as a partial bond is formed involving the nucleophile and carbon atom. Halide and carbon's bond partially dissolves. A transition is created where there are five bonds around the carbon atom. The product is created when the leaving group, which is a halide, removes the electrons from the C-X link and departs the molecule.
R-X + Nu⁻ → Nu
--R--X → Nu-R + X⁻
Since a transition state that contains both a nucleophile and a substrate is used for the nucleophilic substitution. It was discovered that the concentration of the substrate and the nucleophile both affect the rate of the reaction.
Rate = k [substrate] [nucleophile]
Since sodium azide is the nucleophilic species and methyl iodide is the substrate,
Rate = k[CH₃I][NaN₃]
both the reactants take part in the reaction and the rate law for the reaction. As a concentration of NaN₃ is doubled, the new rate becomes,
Rate' = k[CH₃I][2 NaN₃]
= 2 k[CH₃I][NaN₃]
= 2 Rate
Learn more about SN2 mechanism at https://brainly.com/question/29561608
#SPJ4
salt plus water. two compounds mixed together to form a solution. The water is the solvent; the salt is the solution. students have been tasked with separating the two compound from solution.
Answers
Answer:
Evaporation
Explanation:
Students are to mix the solvent to dilute completely,and get and evaporation dish,candle(heat),tripod stand...pour the solution in the evaporation dish and place it on tripod stand above the candle wait for some time the water will change into gas and to get the water they have to cover the evaporating and direct it to a different container to get the water and salt
What is the pH of a solution with an OH- ion concentration of
1.25E-4?
PLEASE HELP ASAAPPOPPP
Answers
Answer:
10.1 M
Explanation:
Applying,
pH = -log(H⁺).................... Equation 1
But,
[H⁺][OH⁻] = 1×10⁻¹⁴................ Equation 2
Where [H⁺] = Hydrogen ion concentration, [OH⁻] = Hydroxyl ion concentration.
From the question,
Given: [OH⁻] = 1.25×10⁻⁴ M
Substitute into equation 2
[H⁺][1.25×10⁻⁴] = 1×10⁻¹⁴
[H⁺] = 1×10⁻¹⁴/1.25×10⁻⁴
[H⁺] = 0.8×10⁻¹⁰ M
[H⁺] = 8×10⁻¹¹ M
Also, Substitute the value of [H⁺] into equation 1
pH = -log[8×10⁻¹¹]
pH = 10.1 M
4. Write the complete electron-configuration
notation, the noble-gas notation, and the orbital
notation for the following elements:
a. carbon b. neon c. sulfur
Answers
a. Carbon:
Complete electron configuration notation: 1s^2 2s^2 2p^2
Noble gas notation: [He] 2s^2 2p^2
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p
b. Neon:
Complete electron configuration notation: 1s^2 2s^2 2p^6
Noble gas notation: [He] 2s^2 2p^6
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p
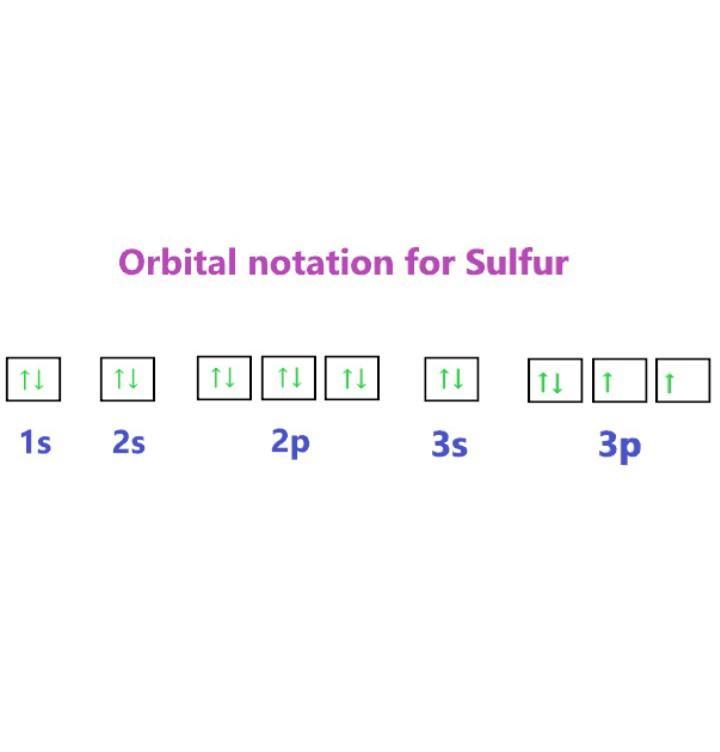
c. Sulfur:
Complete electron configuration notation: 1s^2 2s^2 2p^6 3s^2 3p^4
Noble gas notation: [Ne] 3s^2 3p^4
Orbital notation: ↑↓ 1s ↑↓ 2s ↑↓ 2p ↑↓ 3s ↑↓ 3p
a. Carbon :
Atomic number = 6
Electronic configuration notation : 1s² 2s² 2p²
Noble-gas notation : [He] 2s² 2p²
Orbital notation : Refer to the attachment.
b. Neon
Atomic number : 10
Electronic configuration notation : 1s² 2s² 2p⁶
Noble-gas notation : [He] 2s² 2p⁶
Orbital notation : Refer to the attachment.
c. Sulfur
Atomic number : 16
Electronic configuration notation : 1s² 2s² 2p⁶ 3s² 3p⁴
Noble-gas notation : [Ne] 3s² 3p⁴
Orbital notation : Refer to the attachment.


What is the volume in liters of 423 grams of SO2
Answers
ANSWER
The volume of SO2 is 147.89L
STEP-BY-STEP EXPLANATION:
Given information
\(\text{The volume of SO}_2\text{ is 423 grams}\)Step 1: Find the mole of SO2 using the below formula
\(\text{mole = }\frac{\text{ mass}}{\text{ molar mass}}\)Recall, the molar mass of SO2 is 64.066 g/mol
\(\begin{gathered} \text{mole = }\frac{423}{64.066} \\ \text{mole = 6.603 moles} \end{gathered}\)Recall that, 1 mole of gas is equivalent to 22.4L at STP
Let x represents the volume of SO2
\(\begin{gathered} 1\rightarrow22.4L_{} \\ 6.603\rightarrow\text{ x} \\ \text{cross multiply} \\ 1\text{ }\times x\text{ = 6.603 }\times22.4 \\ x\text{ = 147.89L} \end{gathered}\)Hence, the volume of SO2 is 147.89L
Question 55
Basal and squamous cell carcinomas are most commonly associated with exposure to:
a. Tobacco smoke
b. UVB
c. Nuclear waste
d. Asbestos
Answers
Basal and squamous cell carcinomas are most commonly associated with exposure to UVB radiation, which is present in sunlight.
UVB radiation is a known carcinogen that damages DNA and can lead to skin cancer. Tobacco smoke, nuclear waste, and asbestos are associated with other types of cancer, but not basal and squamous cell carcinomas. Cell carcinomas are a type of cancer that begins in the cells that make up the skin or the lining of organs. There are different types of cell carcinomas, including basal cell carcinoma, squamous cell carcinoma, and transitional cell carcinoma, among others.
Learn more about cell carcinomas here:
https://brainly.com/question/9065487
#SPJ11
Which molecules show an appropriate number of bonds around each carbon atom?.
Answers
Answer:
Draw Lewis structures for the following organic molecules: C2H3F, C3H6C4H8 In each there is one C = C bond, and the rest of the carbon atoms are joined by C - C bonds.
Identify the type of intermolecular force for water, vegetable oil, and corn syrup.
Answers
Answer:
Intermolecular force for oil the dipole-dipole.
Intermolecular force for water hydrogen bonds.
Intermolecular force for vegetable oil nonpolar compound.
Intermolecular force for corn syrup fructose.
Conduct a survey of 5-7 people in your locality to know the usage of plastic bags and awareness to among people regarding their usage
Answers
The usage of plastic bags and the level of awareness among people in your locality. Summarize the findings in a report or presentation, highlighting key findings and any recommendations for promoting sustainable alternatives to plastic bags.
As an AI language model, I don't have direct access to real-time data or the ability to conduct surveys. However, I can provide you with a general framework for conducting a survey on the usage of plastic bags and awareness among people. Here are the steps you can follow:
Define your survey objectives: Determine the specific information you want to gather about the usage of plastic bags and people's awareness. This will help you design appropriate survey questions.
Create survey questions: Develop a set of questions that capture the key aspects you want to investigate. These may include questions about the frequency of plastic bag usage, reasons for using or not using them, knowledge about the environmental impact, and willingness to adopt alternatives.
Determine the sample size: Decide on the number of respondents you want to survey. Aim for a sample size that provides a representative perspective of your locality, but keep in mind the practicalities of reaching out to and collecting responses from the selected participants.
Select participants: Randomly select or identify individuals within your locality to participate in the survey. Consider diversifying the sample to include people of different ages, occupations, and backgrounds for a more comprehensive understanding.
Draw conclusions and report findings: Based on the analyzed data, draw conclusions about the usage of plastic bags and the level of awareness among people in your locality. Summarize the findings in a report or presentation, highlighting key findings and any recommendations for promoting sustainable alternatives to plastic bags.
Learn more about locality here
https://brainly.com/question/32102156
#SPJ1
which gas must not enter the carboy in order for fermentation to occur?
Answers
For fermentation process to successfully occur, Oxygen gas (O2) must not enter the carboy, because the pyruvate used in the process, gets completely oxidized when oxygen gas is present.
Fermentation is a metabolic process that always must take place in the absence of oxygen. Many of the beneficial microorganisms, create desired changes in different types of beverages and foods through this process of fermentation. And the resulting products of fermentation reaction thus formed, have better/more favorable flavor and taste, and also more life as they get preserved during the process. In addition to this, these microorganisms also provide several health benefits.
The process of fermentation to occur successfully, does not require oxygen gas as it is an anaerobic process. If by any chance or means, oxygen gas is present, the pyruvate used would be completely oxidized during the reaction forming carbon dioxide and water molecules as the by products, by the action of yeast spiration. Moreover, these yeast species needed in the reaction, produce ethanol only in an anaerobic (oxygen-less) environment by another process called the Pasteur Effect.
There are generally three types of fermentation processes based on the end products obtained using the pyruvate. They are:
Acetic Acid Fermentation, Lactic Acid Fermentation, and Alcoholic Fermentation.
Therefore, Oxygen Gas is the correct answer to this question.
Learn more about fermentation here:
https://brainly.com/question/13050729
#SPJ4
why should bunsen burners not be used when heating organic materials?
Answers
Answer:
Many organic compounds are very flammable.
Explanation:
Because we do not want to inadvertently ignite chemicals and cause a fire or explosion. Most organic chemicals are incompatible with inorganic chemicals, and certain mixtures can cause violent reactions.
A solution of H2SO4(aq) with a molal concentration of 1.94 m has a density of 1.119 g/mL. What is the molar concentration of this solution?
Answers
The density of H2SO4 is 1.119 g/mL, and the molal concentration is 1.94 m.
We can use the following formula to find the molar concentration of the solution:
Molality (m) = Moles of solute / Mass of solvent in kg
= (mass of solute in grams) / (molar mass of solute × mass of solvent in kg)
the molar mass of H2SO4 as follows:
Molar mass of H2SO4 = 2(1.00794 g/mol of H) + 32.066 g/mol of S + 4(15.9994 g/mol of O)
= 98.079 g/mol
Now, we can find the mass of solvent in kg as follows:
Let's take 1000 g of the solution, and we know that the density of the solution is 1.119 g/mL.
So, 1000 g of solution contains 1000/1.119 = 893.04 mL of the solution, which is equal to 0.89304 L of the solution.
So, mass of solvent in 0.89304 L of the solution = (0.89304 L × 1.119 g/mL) - (mass of solute in 0.89304 L of the solution)
= 1.0 kg (approx)
Now, we can find the number of moles of H2SO4 in 1 kg of the solvent as follows:
Number of moles of H2SO4 = molality × mass of solvent in kg
= 1.94 × 1 kg of solvent
= 1.94 mol
Hence, the molar concentration of the solution is equal to the number of moles of solute per liter of the solution.
Molar concentration of H2SO4 solution = (Number of moles of solute) / (Volume of the solution in liters)
= 1.94 mol / 0.89304 L
= 2.18 M
Therefore, the molar concentration of this solution is 2.18 M (Approx).Thus, the detailed answer to this question has been provided.
Learn more about molal concentration
brainly.com/question/33443849
#SPJ11
An atom has 30 protons, 35 neutrons, and 30 electrons. What is the charge of the atom's nucleus?
Answers
Answer:
+35
Explanation:
the root-mean-square speed of nitrogen molecules in m/s, at 125 oc is closest to...
Answers
The root-mean-square (rms) speed of nitrogen molecules can be calculated using the formula vrms = √(3kT/m), where k is Boltzmann's constant, T is the temperature in Kelvin, and m is the mass of one nitrogen molecule. At 125°C, which is 398 K, the vrms of nitrogen molecules is closest to 585 m/s.
To arrive at this answer, we need to convert the temperature to Kelvin (398 K) and the mass of a nitrogen molecule is 28 atomic mass units. Using these values and the formula, we can calculate the vrms of nitrogen molecules to be 585 m/s.
The root-mean-square speed (RMS speed) of nitrogen molecules at 125°C can be calculated using the formula:
RMS speed = √(3RT/M)
where R is the ideal gas constant (8.314 J/(mol·K)), T is the temperature in Kelvin (125°C + 273.15 = 398.15 K), and M is the molar mass of nitrogen (28.02 g/mol x 0.001 kg/g = 0.02802 kg/mol).
Plugging these values into the formula:
RMS speed = √(3 × 8.314 × 398.15 / 0.02802)
RMS speed ≈ 515 m/s
So, the root-mean-square speed of nitrogen molecules at 125°C is closest to 515 m/s.
To know about RMS visit:
https://brainly.com/question/29662026
#SPJ11
In a combustion reaction, how many liters of oxygen gas are required to fully react with 80 g of pentane gas? Stoichiometry.
Answers
Answer:
26.4 L
Explanation:
O₂ + C₅H₈
Given: 80 g of C₅H₈
Find actual gram-formula mass of C₅H₈: 68 g
Create a proportion: 80 g/68 g = 1.176…
Find actual gram-formula mass of O₂: 16 g
Find new mass of O₂: 16 g * 1.176… = 19 g
Use dimensional analysis for conversion (see attachment).

State the worded equation for the reaction that occurred in a beaker when copper is placed in a beaker of silver nitrate.
Answers
The reaction of silver nitrate with copper is a substitution reaction. We have copper in its free state, Cu, and we have silver nitrate which has the formula AgNO3. The copper replaces the silver obtaining the following reaction:
\(2AgNO_{3(aq)}+Cu_{(s)}\rightarrow Cu(NO_3)_{2(aq)}+2Ag_{(s)}\)The products obtained are copper nitrate and silver.
The word equation will be:
\(SilverNitrate+Copper\rightarrow Copper(II)Nitrate+Silver\)Using standard reduction potentials from the aleks data tab, calculate the standard reaction free energy for the following redox reaction. 3fe2 cro42- 4h20
Answers
The standard Gibbs free energy for the given redox reaction is -106kJ/mol.
What is the standard Gibbs free energy?
The standard Gibbs free energy (ΔG°) is a thermodynamic potential that can be used to calculate the maximum reversible work that may be performed by a thermodynamic system under standard conditions.
To find the Gibbs free energy, first we have to know about the redox reaction,
10 Cl⁻(aq) + 2 MnO₄⁻(aq) + 16 H⁺(aq) ⇒ 5 Cl₂(g) + 2 Mn²⁺(aq) + 8 H₂O(l)
As we have to analyze the standard reduction potentials (E°red).
Cathode: 2 MnO₄⁻ + 16 H⁺ + 10 e⁻ ⇒ 2 Mn²⁺ + 8 H₂O
E°red = 1.51 V
Anode: 10 Cl⁻ ⇒ 5 Cl₂ + 10 e⁻
E°red = 1.40 V
Then, the potential difference between the cathode and anode is called the standard cell potential.
E°cell = E°red, cathode - E°red, anode
= 1.51 V - 1.40 V
= 0.11 V
The standard cell potential is 0.11 V.
The Gibbs free energy can be calculated as,
ΔG° = - n × F × E°cell
ΔG° = - 10 mol × (96,485 J/V.mol) × 0.11 V × (1 kJ/1000 J)
= -106 kJ/mol
where,
n - moles of electrons exchanged.
F -Faraday's constant.
The standard Gibbs free energy for the given redox reaction is -106kJ/mol.
Learn more about Standard Gibbs free energy, https://brainly.com/question/9238751
#SPJ4
Does a hypothesis explains what the scientist thinks will happen during the experiment.
Answers
Hope this helps!
What are the astronomer qualifications
Answers
Minimum qualifications of Astronomer 4 year degree to get a postgraduate qualification like a master of physics.
2 or 3 A levels, or similar, in math and physics; 5 GCSEs at grades 9 to 4 (A* to C), or equivalent; and a degree in a field that is useful for postgraduate study.
Math and physics expertise, analytical thinking abilities, science knowledge, great verbal communication skills, the capacity to take initiative, and the capacity to think coherently utilizing logic and reasoning are all desirable. To be extremely knowledgeable about computer applications and systems.
A scientist who concentrates their research on a particular issue or area outside the purview of Earth is called an astronomer in the science of astronomy. In either observational or theoretical astronomy, they observe celestial bodies like stars, planets, moons, comets, and galaxies.
To learn more about Astronomer, refer this link.
https://brainly.com/question/1003405
#SPJ1
Determine the dimensions of 7 . which is the viscosity of a liquid, by performing dimensional analysis of the following equation. F=2πrL
R
v
, eetc F is force (Kgm/s
2
) r is radius (m) L is length (m) v is speed (m/s) R is distance (m)
Answers
The dimensions of viscosity are kilograms per meter per second (kg/(m·s)).
To determine the dimensions of viscosity (symbolized as η), we can perform dimensional analysis on the given equation:
F = 2πrL / Rv
Breaking down the dimensions of each variable:
F: Force, [M][L][T]⁻²
r: Radius, [L]
L: Length, [L]
R: Distance, [L]
v: Speed, [L][T]⁻¹
Substituting the dimensions into the equation:
[M][L][T]⁻² = 2π[L][L][L] / [L][L][T]⁻¹ * η
Simplifying the equation:
[M][L][T]⁻² = 2π[L]⁴[T] * η
Equating the dimensions on both sides of the equation:
[M] = 2π[L]³[T]² * η
From this equation, we can see that the dimensions of viscosity (η) are:
[η] = [M][L]⁻¹[T]⁻¹
Therefore, the dimensions of viscosity are kilograms per meter per second (kg/(m·s)).
Learn more about viscosity from the given link
https://brainly.com/question/2568610
#SPJ11
alkanes are hydrocarbons containing only single bonds. how many hydrogen atoms are in an acyclic alkane with 5 carbon atoms?
Answers
The total number of Hydrogen contained by the hydrocarbon alkane having 5 carbon atoms will be 12.
Alkanes are saturated hydrocarbons having molecular formula ( CnH₂n +2).
So, on writing the molecular formula of a hydrocarbon containing only 5 Carbon atom and the straight chained is written as follows:
CH₃CH₂CH₂CH₂CH₃
and the name of this hydrocarbon compound will be the pentane according to IUPAC system of nomenclature.
Calculating the total number of Hydrogens atoms
There are 12 H atoms in total
If we calculate it through the general formula of the molecule of alkane ,we get, ( CnH₂n +2)
where,
n= number of C atoms = 5
C₅H₅ₓ₂ + 2 = C₅H₁₂
Number of hydrogen= 12
To know more about hydrocarbons, please refer;
https://brainly.com/question/3551546
#SPJ4
MAGNESIO + OXIGENO
nombre
formula
Answers
Answer:
Óxido de magnesio
Concepto:Es un óxido metálico, formado por magnesio y oxígeno, de estructura iónica cuya fórmula química es MgO.
Blue bowling ball rolled with a force of 15 N accelerates at a rate of 3 m/sec2 a second red ball rolled with the same force accelerates at 4 m/sec2. What are the masses of the two balls?
Answers
Answer:
1. Mass of the blue ball is 5 kg.
2. Mass of the red ball is 3.75 kg.
Explanation:
The following data were obtained from the question:
For the blue ball:
Force (F) = 15 N
Acceleration (a) = 3 m/s²
Mass (m) =.?
Force the red ball:
Force (F) = the same with that of the blue ball = 15 N
Acceleration (a) = 4 m/s²
Mass (m) =.?
1. Determination of the mass of the blue ball.
Force (F) = 15 N
Acceleration (a) = 3 m/s²
Mass (m) =.?
Force (F) = mass (m) × acceleration (a)
F = ma
15 = m × 3
Divide both side by 3
m = 15/3
m = 5 kg
Therefore, the mass of the blue ball is 5 kg
2. Determination of the mass of the red ball.
Force (F) = 15 N
Acceleration (a) = 4 m/s²
Mass (m) =.?
Force (F) = mass (m) × acceleration (a)
F = ma
15 = m × 4
Divide both side by 4
m = 15/4
m = 3.75 kg
Therefore, the mass of the blue ball is 3.75 kg
Determine whether each statement describes a solution of a strong electrolyte, weak electrolyte, or non-electrolyte.
Answers
Please find the terms with their correct description in the explanation section.
In an aqueous solution, an electrolyte is a material that separates or ionizes into cations (positively charged ions) and anions (negatively charged ions). Depending on how well it ionizes and how much conductivity it has, an electrolyte can be either strong or weak. A non-electrolyte, however, does not conduct electricity or ionize in a solution.
Based on this;
1. Weak electrolyte: Has a medium level of conductivity i.e. partially conducts electricity
2. Strong electrolyte: Contains a complete solute
3. Non-electrolyte: Has little or no conductivity i.e. cannot conduct electricity because it doesn't dissociate into ions.
4. Strong electrolyte: Has the highest conductivity i.e. conducts electricity very well.
5. Strong electrolyte: Contains a completely dissociated solute i.e. the solute of the electrolyte separates into anions and cations completely.
6. Weak electrolyte: Contains a partially dissociated solute i.e. the ions of the solute do not ionize completely in the solution.
To learn more about electrolyte visit:https://brainly.com/question/28699046
#SPJ1
Why do non-metals bond with each other to form covalent compound?
Answers
Answer:
Explanation:
Because unlike ionic bonds (metal and a non-metal) where electrons are "stolen", covalent bonds are defined by the sharing of electrons between non-metals.
How many atoms of carbon present in 3.9g of benzene
Answers
Answer:
no. of carbon atoms = 1.806 × 10²³
Explanation:
The question asks us to find the number of atoms in 3.9 g of benzene.
To do this we must know that the chemical formula of benzene is C₆H₆.
Therefore, the molecular mass of benzene is:
R.M.M = (12 × 6) + (1 × 6)
= 72 + 6
= 78
Now that we know the molecular mass of benzene, we have to find the number of moles of benzene in 3.9 g of benzene:
no. of moles = \(\mathrm{\frac{mass}{R.M.M}}\)
= \(\frac{3.9}{78}\)
= 0.05 mol
From the number of moles, we can find the number of molecules of benzene using the formula:
\(\boxed{\mathrm{no. \ of \ molecules = no. \ of \ moles \times Avogadro's \ number}}\)
where Avogadro's number = 6.02 × 10²³
Therefore,
no. of molecules of benzene = 0.05 × 6.02 × 10²³
= 3.01 × 10²²
Since each molecule of benzene contains 6 atoms of carbon, we have to multiply the number of benzene molecules by 6:
no. of carbon atoms = 3.01 × 10²² × 6
= 1.806 × 10²³
Therefore, there are 1.806 × 10²³ atoms of carbon in 3.9 g of benzene.