What process is demonstrated by an acid reacting with a base?
Answers
The process demonstrated by an acid reacting with a base is called neutralization. In this reaction, the acid and base combine to form salt and water.
Neutralization is a reaction between an acid and a base that results in the formation of a salt and water. When an acid and a base are mixed together, the acid donates a proton (H+) to the base, forming water and an ionic compound called a salt. The reaction is a double displacement reaction, in which the hydrogen ions (H+) from the acid combine with the hydroxide ions (OH-) from the base to form water (H₂O). The remaining ions then combine to form a salt.
For example, the reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is a classic example of a neutralization reaction.
The reaction is as follows: HCl + NaOH → NaCl + H₂O
In this reaction, the hydrogen ions (H+) from the hydrochloric acid combine with the hydroxide ions (OH-) from the sodium hydroxide to form water (H₂O). The remaining ions (Na+ and Cl-) combine to form the salt sodium chloride (NaCl).
Learn more about "Acid"; https://brainly.com/question/8743072
#SPJ11
Related Questions
What is the freezing point of neon?
Answers
248.4 °C
Explanation:
Boiling point: -246 °C
Atomic mass: 20.1797 u
Symbol: Ne
Cadmium (Cd) is a solid at room temperature, forms an ion that has a +1 or +2 charge, is a less reactive metal, and forms more than one unique compound with a given nonmetal. Because of these properties, cadmium (Cd) could also be identified as
a halogen.
an alkaline earth metal.
a transition metal.
an alkali metal.
Answers
Because of these properties, cadmium (Cd) could also be identified as a transition metal.
Cadmium (Cd) is a solid at room temperature, forms an ion that has a +1 or +2 charge, is a less reactive metal, and forms more than one unique compound with a given nonmetal.
Transition metal, any of various chemical factors which have valence electrons i.e., electrons that can participate inside the formation of chemical bonds in two shells as opposed to simplest one.
Transition metals are determined within the periodic desk among the s-block and p-block elements. Hence, they are known as d-block elements. Transition metals are unstable metals that display transitional conduct between s and p block elements, therefore their name.
Learn more about transition metal here:- https://brainly.com/question/2879928
#SPJ1
a solid has a mp of 133-137 oc. what can one conclude about the sample? the sample is one of four possible compounds the
Answers
A solid sample has a MP of 133 - 137°C. one can conclude about the sample is It is relatively impure.
the temperature at which a compound completely melts is called as melting point but generally we don't observe a melting point rather a range is observed if range more than 20 C is observed generally it's said that it's impure an impure solid melts over a wide range and at a temperature lower than that of the pure solid. so since the last value 139 0 C must be the reported value when you must have seen it completely melting so it's melting point should be this only .
learn more about solid temperatures here:
https://brainly.com/question/2160033
#SPJ4
does Waves move in a circular motion.
True or false
Answers
why yes
Waves are created by energy passing through water, causing it to move in a circular motion
imagine you have collected data on the temperature and dissolved oxygen in a eutrophic lake over five years. you want to see if dissolved oxygen varies with temperature in this lake. what statistical test would you do ? explain why you chose this test
Answers
We can make use of the correlation approach, T test, and linear regression techniques in this situation.
The two variables in this example of a eutrophic lake are temperature and dissolved oxygen, and both of these variables change over time. Therefore, a correlation test must be performed to see whether a relationship between these two variables exists. Here, the correlation coefficient value will be given to us, allowing us to calculate the linear relationship between these two variables.
Additionally, we can use the T test to determine whether or not the correlation between these two variables is strong enough to reject the null hypothesis.
Finally, we can also utilize the linear regression method to get a more accurate relationship between those two variables.
Learn more about Hypothesis here:
https://brainly.com/question/12687557
#SPJ4
IS IT BETTER OR WORSE TO WORKOUT/EXERCISE IN DARK OR LIGHT COLORED CLOTHING?
Answers
Answer:
Dark clothing
Explanation:
Dark clothing like black absorbs/locks in heat. Since exercise is to burn and tonify our body from unwanted fat it is important to sweat. This is where heat comes in and helps you sweat
I prefer light coloured cloth to wear, as if we wear dark coloured cloth during exercise it will absorb more heat bcox of the thickness and etc etc..but when it's light coloured cloth it won't have much thickness and it's fabric is just suitable for warm places..
What is the activation energy of a reaction?
Answers
Answer:
The activation energy for the forward reaction is the amount of free energy that must be added to go from the energy level of the reactants to the energy level of the transition state. ... Once a reactant molecule absorbs enough energy to reach the transition state, it can proceed through the remainder of the reaction.
Explanation:
Answer: Activation energy is how much energy it takes for a reaction to occur.
Explanation:
Which is the least reactive compound by the sn1 mechanism? a. ch3ch2ch2ch2br b. (ch3)2chch2br
Answers
The compound (CH3)2CHCH2Br is the least reactive compound by the SN1 mechanism among the options provided. This is due to the increased stability of the carbocation intermediate formed during the SN1 reaction, which is influenced by the presence of alkyl groups.
The SN1 mechanism involves a two-step process: the formation of a carbocation intermediate followed by the nucleophilic attack. In this case, we are comparing two compounds: CH3CH2CH2CH2Br (option a) and (CH3)2CHCH2Br (option b).
In option a, CH3CH2CH2CH2Br, the carbon attached to the bromine (the reaction center) is a primary carbon, meaning it has only one alkyl group attached to it. Primary carbocations are highly unstable due to the lack of nearby alkyl groups to stabilize the positive charge. As a result, the formation of the carbocation intermediate is less favorable, making this compound more reactive via the SN1 mechanism.
In option b, (CH3)2CHCH2Br, the carbon attached to the bromine is a tertiary carbon, meaning it has three alkyl groups attached to it. Tertiary carbocations are more stable than primary carbocations due to the presence of nearby alkyl groups, which can donate electron density and stabilize the positive charge. Therefore, the formation of the carbocation intermediate is more favorable, making this compound less reactive via the SN1 mechanism.
In summary, (CH3)2CHCH2Br is the least reactive compound by the SN1 mechanism because the tertiary carbocation intermediate formed is more stable compared to the primary carbocation intermediate in CH3CH2CH2CH2Br.
Learn more about nucleophilic attack here:
brainly.com/question/28325919
#SPJ11
in order to have a more observable reaction, a student decides to add some deionized water to the erlenmeyer flask. describe how the experimental molarity of \text{h}\text{cl} will be skewed.
Answers
The experimental molarity of HCl will be skewed, causing the observed reaction to potentially be less intense or slower than it would have been at the original molarity.
In order to address your question about how the experimental molarity of HCl will be skewed when a student adds deionized water to the Erlenmeyer flask, consider the following:
1. The student has an initial molarity (concentration) of HCl in the Erlenmeyer flask.
2. By adding deionized water to the flask, the student increases the total volume of the solution.
3. Molarity is defined as the number of moles of solute (in this case, HCl) per liter of solution.
When the volume of the solution increases due to the addition of deionized water, the molarity (concentration) of HCl will decrease. This is because the number of moles of HCl remains the same, but the total volume of the solution has increased.
Learn more about molarity : https://brainly.com/question/30404105
#SPJ11
Suppoe that you ue 4. 25 g of Iron in the chemical reaction: 2Fe() 3Cu2(aq) rightward arrow 2Fe3(aq) 3Cu(). What i the theoretical yield of Cu(), in gram?
Answers
Theoretical yield of copper is 7.22 g when 4.25g of iron in the chemical reaction.
2Fe(s) + 3Cu2(aq.) ----> 2Fe3(aq.) + 3Cu (s)
Molar mass of Iron (Fe) is 56g/mole.
Molar mass of copper (cu) is 63.5g/mole.
2 mole ( 2 * 56g/mole) Iron produces = 3 mole ( 3 * 63.5g/mole ) copper
Theoretical yield is the maximum amount of product that could be formed from the given amounts of reactants. The actual yield is the amount of product that is actually formed when the reaction is carried out in the laboratory. The theoretical yield is the amount of product that would be formed from a reaction if it was 100% efficient.
There are 4. 25 g of Iron .so,
4.25 g Iron produces = (3 * 63.5g / 2 * 56 g ) * 4.25 g copper
= 7.22 g copper
Theoretical yield of copper is 7.22g.
To learn more about Theoretical yield please visit:
https://brainly.com/question/2765357
#SPJ4
The experiment set-up shown in the picture has a light-proof box with a small hole on one side. What will be the shape of the image of the arrow on the opposite wall?
(The arrow is pointing up in the image of the arrow, also the box has a hole on it's left side.)
A. Left
B. Up
C. Down
D. Right
Answers
Answer: A.
Explanation: When light passes through a small hole, it creates an inverted image on the opposite side. In this case, since the arrow is pointing up, the inverted image will appear pointing down on the opposite wall. Furthermore, since the box has a hole on its left side, the inverted image will be shifted towards the left.
A slightly edited Exercise 6 of Chapter 4 (Page 90) states:
(a) Calculate the energy needed to bring a cup of water (about 250 g) from 10°C to the boiling point (100°C for water). Then, find the time it takes to heat this water (c) in a 1-kg aluminum pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point.
Solution:
(a) To heat just the water requires energy Qw=mwcwΔT (Equation 4.3), where ΔT=100∘C−10∘C=90∘C:
Qw=0.25kg(4184Jkg∘C)90∘C=94,140J
(c) On the stove, we also have to heat the pan. Aluminum’s specific heat is ca=900Jkg∘C , from table 4.3, (because this is lower than cw, it is easier to heat aluminum than water).
To heat just the aluminum pan requires energy, Qa=macaΔT=1kg(900Jkg∘C)90∘C.
The total energy to heat the pan of water on the stove is increased because of the finite efficiency:
Qtotal=Qw+Qaes=94,140J+81,000J0.75=233,520J
The time it takes to heat the water depends on the stove’s power: power = energy per time, so
t=energypower=QtotalPs=233,520J1,500Js=155.68or156sonthestove
Question:
Find the time, in seconds, it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. Assume the pan, too, starts at 10°C and has to be heated to water’s boiling point. Round your answer to the nearest whole second.
Answers
The time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
We need to calculate the time taken to heat the water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan. The given information are as follows:
Specific heat of water, cw = 4184 J/kg °C
Specific heat of steel, cs = 450 J/kg °C
Energy supplied by the electric stove burner, P = 1,500 W (75% of which is transferred to the water and the pan)
Mass of water, mw = 250 g = 0.25 kg
Mass of steel pan, ms = 1 kg
Initial temperature of water and steel pan, T1 = 10 °C
Final temperature of water and steel pan (boiling point of water), T2 = 100 °C
Heat absorbed by the steel pan = Qs = ms × cs × (T2 - T1)Heat absorbed by the water = Qw = mw × cw × (T2 - T1)
Total heat absorbed by the water and the pan = Q = Qw + Qs = (0.25 × 4184 × 90) + (1 × 450 × 90) J= 94,140 + 40,500 J= 1,34,640 J
Time taken to heat the water and the pan = t = Q/P= 1,34,640 / 1,500 s= 89.76 or 90 s
Therefore, the time it takes to heat this water in a 1-kg steel pan sitting on a 1,500-W electric stove burner that transfers 75% of its energy output to the water and the pan is 90 seconds (rounded to the nearest whole second).
To know more about Mass, visit:
https://brainly.com/question/11954533
#SPJ11
The temperature of a 0.65L sample of carbon dioxide gas is 580K. If the pressure remains constant, what is the new volume of the gas if the temperature increases to 1300K?
Answers
what functional groups are involved in forming a peptide bond
Answers
The functional groups that are involved in forming a peptide bond are the amine group (-NH2) and the carboxyl group (-COOH).
The formation of a peptide bond involves two functional groups: the carboxyl group (-COOH) of one amino acid and the amino group (-NH2) of another amino acid. During the process, a condensation reaction occurs, resulting in the release of a water molecule. The carboxyl group of one amino acid donates a hydrogen atom (H) to the amino group of the adjacent amino acid, creating a peptide bond and forming a dipeptide. This process can continue through multiple amino acids, leading to the formation of longer polypeptide chains. Peptide bonds are crucial for the structural and functional integrity of proteins in living organisms. Proteins, which are made up of one or more polypeptide chains, are the most common type of biomolecules in living organisms.
To know more about dipeptide, visit:
https://brainly.com/question/32240062
#SPJ11
In what form is a group when pH is greater than pKa?
Answers
When the pH is greater than the pKa, the group is in its deprotonated form.
The pKa is a measure of the acidity of a compound, and specifically refers to the pH at which half of the molecules in a solution are in their protonated form and half are in their deprotonated form. When the pH of a solution is greater than the pKa of a group, this means that the concentration of hydrogen ions in the solution is lower than the concentration of the protonated form of the group, and so the group will tend to lose a hydrogen ion and become negatively charged.
The pKa value represents the pH at which a group is half protonated and half deprotonated. When the pH is greater than the pKa, the group will lose its proton and become deprotonated due to the more basic (alkaline) environment.
To know more about deprotonated visit:-
https://brainly.com/question/31554829
#SPJ11
What is [Al(H2O)5(OH) 2+] in a 0. 15 M solution of Al(NO3)3 that contains enough of the strong acid HNO3 to bring [H3O +] to 0. 10 M?
Answers
Al(NO3)3 solution concentration and the concentration of H3O+ ions in the solution following the addition of HNO3 are given in the problem. We can determine the presence of [Al(H2O)5(OH)2+] in the solution using this knowledge along with the known equilibria for the hydrolysis of Al3+.
For Al3+, the hydrolysis process may be expressed as follows:
Al(H2O)63+ + water becomes Al(H2O)5(OH)2+ + H3O+.
The reaction's equilibrium constant expression is as follows:
Al(H2O)5(OH)2+) = K
Al(H2O)63+ / [H3O+]
We must take into account the dissociation of Al(NO3)3 in water in order to determine [Al(H2O)5(OH)2+] in a 0.15 M solution of Al(NO3)3:
Al3+ (aq) + 3NO3- Al(NO3)3 (s) (aq)
Al3+ has a concentration of 0.45 M (3 times that of the Al(NO3)3 solution) in an Al(NO3)3 solution with a concentration of 0.15 M. H3O+ is present in the solution at a concentration of 0.10 M.
learn more about Al(NO3)3 solution here:
https://brainly.com/question/14215622
#SPJ4
20. What are the metals in the center of the periodic table referred to as?
Answers
The metals in the center of the periodic table are called transition metals. There are 38 transition metals in total.
Answer:
They are called transition metals.
CuSO4 . 5H2O is dehydrated to form Copper (ii) sulfate. Is this a physical or chemical change? Explain.
Answers
Answer:
It is a physical change.
Explanation:
• This is because when anhydrated copper (ii) sulfate is heated, the water evaporates to form anhydrous copper (ii) sulfate.
• evaporation is a physical change
\(.\)
What happens to valence electrons during a covalent bond?
Answers
Answer:
Covalent bonding occurs when pairs of electrons are shared by atoms. Atoms will covalently bond with other atoms in order to gain more stability, which is gained by forming a full electron shell. By sharing their outer most (valence) electrons, atoms can fill up their outer electron shell and gain stability.
Is the pattern of atomic radius absolute or general (always true or generally true)?
Answers
Answer:
Generally true.
Explanation:
As you go down the columns of the Periodic Table, the radius of the atom increases.
As you go across the Periodic Table (left to right), the radius of the atoms decrease.
Therefore, the second element on the table, Helium, has the smallest radius and element 87, Francium has the largest radius.
There are a few exception scattered throughout the table, such as the lanthanides and actinides, but generally speaking the radius increases as you travel from right to left and top to bottom.
Earth’s spheres interact in a system that makes up all of the matter, energy, and processes within Earth’s boundary. You learned about the different spheres in this lesson and how spheres are not isolated, but they interact. In the space below, explain how the biosphere relies on the other spheres for survival.
Answers
Answer:
Explanatthe tryposphereion:
Which of the following is a solid fuel (multicorrect)
(a)Petrol
(b)Diesel
(c) Wax
(d)Coal
Answers
Answer:
the answer is coal
Explanation:
Volume of 22mm x 15 mm x 2.0 mm
Answers
The formula for the volume of a rectangular solid is Volume = length * width * height, or V = lwh.
\(volume = 22mm \times 15mm \times 2.0mm \\ = 22mm \times 30mm \\ = 660mm \\ \)Converting to cm = \( \frac{660}{10} = 66 {cm}^{3 } \\ \)
- BRAINLIEST answerer
The stock solution of hydrochloric acid is 12.0M HCI. If the teacher starts with 130 ml of this concentrated acid, what volume of 3.0M HCI can be prepared?
Answers
Answer:
520mL
Explanation:
Data obtained from the question include:
Molarity of stock solution (M1) = 12M
Volume of stock solution (V1) = 130mL
Molarity of diluted solution (M2) = 3M
Volume of diluted (V2) =..?
The volume of the diluted solution can be obtained as follow:
M1V1 = M2V2
12 x 130 = 3 x V2
Divide both side by 3
V2 = 12 x 130 / 3
V2 = 520mL.
Therefore, 520mL of the diluted solution can be prepared.
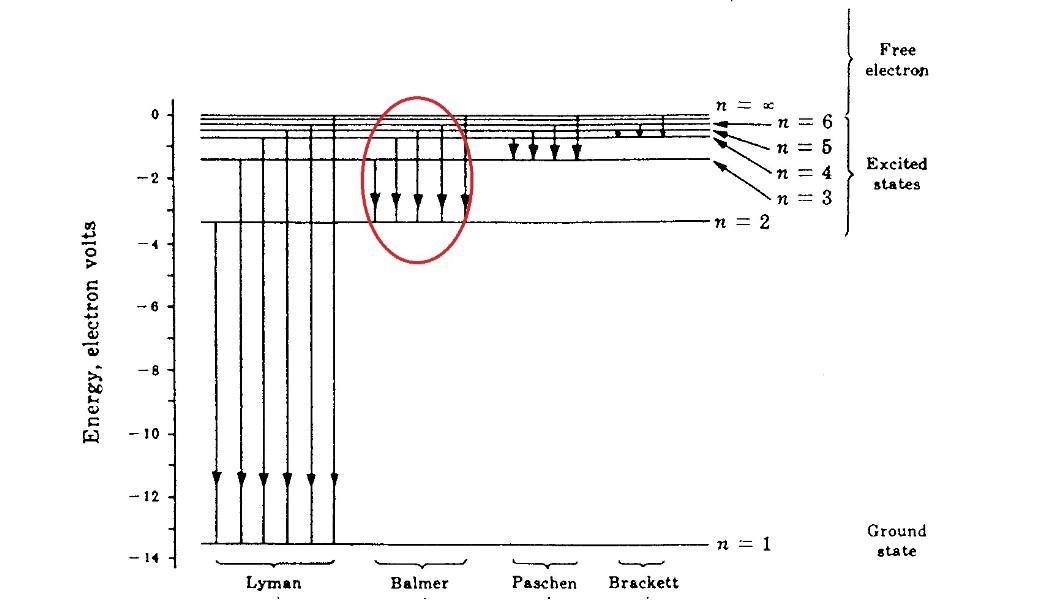
how would the wavelength of the emitted or absorbed photon compare to that of the photon involved in transition c?
Answers
The wavelength, measured in nanometers (nm), of a photon released during the hydrogen atom's transition from the n = 5 state to the n = 2 state is λ = 433.9367 n m
What is meant by wavelength?Wavelength is the separation between identical points (adjacent crests) in successive cycles of a waveform signal traveling through space or over a wire. In wireless systems, this length is typically expressed in meters (m), centimeters (cm), or millimeters (mm).Wavelength is typically represented by the Greek letter lambda (), and it is equal to the ratio of the speed (v) of a wave train in a medium to its frequency (f): = v/f. Wavelength may be computed using the following equation: wavelength = wave velocity/frequency. In most cases, wave length is measured in meters. Greek letter lambda is the sign for wavelength, therefore = v/f. Therefore, the SI unit for wavelength is the Meter (m).Given,
\($\lambda=\frac{h c}{E_p}\)
Ep is the energy of photon.
Since, \($E_p=\frac{2 \pi^2 m_e K^2 Z^2 e^4}{h^2}\left(\frac{1}{n_2^2}-\frac{1}{n_5^2}\right)$\)
\($& K=\frac{1}{4 \pi \varepsilon_0}, n_2=2, n_5=5, Z=1 \\\)
\($& \lambda=\frac{800 \cdot h^3 \cdot c \cdot \varepsilon_0^2}{21 \cdot m_e \cdot e^4}\)
Putting in the values of physical constants
\($\lambda=433.9367 n m\)
Here, λ wavelength of photon
h = Planck's constant
me mass of electron
c = speed of light in vacuum
e = elementary charge
Z = Atomic No. [1 for hydrogen]
The complete question is:
What is the wavelength, in nm, of a photon emitted during a transition from the n = 5 state to the n = 2 state in the hydrogen atom ?
To learn more about wavelength, refer to:
https://brainly.com/question/28995449
#SPJ4
What do an engine using gasoline to power a car and
mixing glue and laundry powder to create putty have in commen
Answers
An engine using gasoline to power a car and mixing glue and laundry powder to create putty are both examples of chemical reactions.
Gasoline is considered an energy source due to its ability to release stored chemical energy in the form of heat and mechanical work when it is burned in an engine. When gasoline is ignited in an engine, the chemical energy stored in its molecular bonds is released, causing a rapid combustion reaction that generates heat and expanding gases that push the pistons and create mechanical work.
The energy content of gasoline is typically measured in units of joules or British thermal units (BTUs), which are used to quantify the amount of energy released during combustion. Gasoline is a widely used and important energy source, but its combustion also produces harmful emissions that contribute to air pollution and climate change.
To learn more about Gasoline visit here:
brainly.com/question/29991915
#SPJ4
what is elastic energy? list two examples of where it is found?
Answers
Sling shot, Bow and arrow
elastic energy is like a rubber band kind of
hope this helped
A sample of certain gas have Volume of 1.25 L ATM _125 degree Celsius and5.0 ATM the gas is compressed 50.0 ATM a volume of 325 mL. what is final temperature?
Answers
The final temperature of the gas is approximately 40.96 Kelvin.
To determine the final temperature of the gas, we can use the ideal gas law, which states:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles of the gas, R is the ideal gas constant, and T is the temperature in Kelvin.
First, let's convert the given temperatures to Kelvin. We have:
Initial temperature: -125 degrees Celsius = 148 K (approximate)
Final temperature: Unknown
The initial conditions of the gas are as follows:
Initial pressure (P1) = 1.25 atm
Initial volume (V1) = 1250 mL = 1.25 L (since 1 L = 1000 mL)
Initial temperature (T1) = 148 K
The final conditions of the gas are as follows:
Final pressure (P2) = 50.0 atm
Final volume (V2) = 325 mL = 0.325 L
Final temperature (T2) = Unknown
Using the ideal gas law, we can set up the following equation:
(P1 * V1) / T1 = (P2 * V2) / T2
Substituting the known values:
(1.25 atm * 1.25 L) / 148 K = (50.0 atm * 0.325 L) / T2
Simplifying the equation:
T2 = (50.0 atm * 0.325 L * 148 K) / (1.25 atm * 1.25 L)
T2 = 40.96 K
For more such questions on temperature visit;'
https://brainly.com/question/4735135
#SPJ8
A solution of NaCl was prepared in the following manner: 0.0842 g of NaCl is massed out on an analytical balance. The solid is transferred to a 25.00 mL volumetric flask. Deionized water is added to the flask such that the bottom of the meniscus is at the line. A 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask using a volumetric pipet and diluted to volume. 6. Calculate the concentration of NaCl in the resulting solution in mg/L NaCl. (answer = 67.4 mg/L) 7. Calculate the concentration of NaCl in the resulting solution using propagation of error through the calculation. Use the manufacturer's tolerance values as the absolute error. The tolerances can be found in Chapter 2 of the Harris text. Assume a Class 1 balance and Class A glassware. Treat the tolerances as random error. (answer = 67.4+0.4 mg/L) 8. Identify 2 possible sources of random (indeterminate) error. Identify 2 possible sourses of systematic (determinate) error.
Answers
Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl
To calculate the concentration of NaCl in the resulting solution in mg/L NaCl, we can use the formula; Concentration (mg/L) = (Mass of solute ÷ Volume of solution in L) × 1000 g / 1 mg NaCl is present in the stock solution of 25 mL. So, the mass of NaCl in the solution would be;0.0842 g ÷ 25 mL = 0.00337 g/mL. Now, in the resulting solution, a 1.00 mL aliquot of the stock solution is transferred to a 50.00 mL volumetric flask and diluted to volume. Therefore, the volume of the resulting solution is 50.00 mL. We will substitute these values in the formula, Concentration (mg/L) = (0.00337 g/mL ÷ 50 mL) × 1000 g / 1 mg concentration (mg/L) = 67.4 mg/L. Therefore, the concentration of NaCl in the resulting solution in mg/L NaCl is 67.4 mg/L.7. Concentration = 67.4 mg/LTolerance = 4.28 mg/LTotal concentration = 67.4 + 4.28 mg/L = 71.68 mg/LWe round off this value to one decimal place; Total concentration = 71.7 mg/LTherefore, the concentration of NaCl in the resulting solution using propagation of error through the calculation is 67.4+0.4 mg/L.8. Two possible sources of random (indeterminate) error in the experiment are; Errors in temperature measurement. Errors in measurement of water volume. Two possible sources of systematic (determinate) error in the experiment are; Incorrect calibration of volumetric glasswareIncorrect mass of NaCl.
Learn more about NaCl
https://brainly.com/question/32275922?
#SPJ11
In a complete sentence, write down a method you could use to determine if an equation is written in the correct way.
Answers
Answer:
An equation is written in a correct way if the equation is balanced. If you want to find out if the equation is balanced, you have to count how many molecules of each element is present and then just balance the equation on the left side and the right side.