Answers
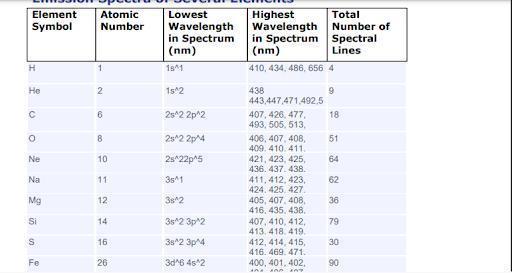
As we continue to increase the available energy levels as shown by the principal quantum number and more electrons are added, the number of lines in the emission spectrum continues to increase.
What is a spectrum?Following the Bohr model of the atom, We could have electrons travel from a higher to a lower energy level . Thus ow leads to the emission of light of a characteristic wavelength. This line is what constitutes the emission spectrum of an atom.
If is clear from the image attached to this answer that as we continue to increase the available energy levels as shown by the principal quantum number and more electrons are added, the number of lines in the emission spectrum continues to increase.
Learn more about spectrum:brainly.com/question/3997802
#SPJ1
Related Questions
When a hydrogen atom is added to a polyatomic ion, the amount of negative charge . Following this pattern, we can see that hydrogen carbonate has a charge of and hydrogen sulfate has a charge of .
Answers
If we add one or two hydrogen ions to a polyatomic ion that has a 3-charge, as the phosphate ion (PO₄3-), it will still be a polyatomic ion. (Three H+ would entirely cancel out the 3-charge, turning it into a neutral molecule and removing it from the category of polyatomic ions.
Why does carbonate have a negative 2 charge?As a result, the carbonate ion has 2 more electrons than protons due to its negative charge. The doubly bonded oxygen in the carbonate ion is neutral, whereas each single bonded oxygen has a negative charge. This is the cause of the total charge of "-2," then.
An essential component of the atmosphere of stars like the Sun is the hydrogen anion.
learn more about carbonate ion
https://brainly.com/question/28770987
#SPJ1
What is the density of an unknown compound in g/ml if 1.28 pounds of the compound has a volume of 4.50L
Answers
1.28 pounds * 453.59 grams/pound = 580.61 grams
Next, we can use the formula for density:
Density = Mass / Volume
Density = 580.61 grams / 4.50 L
Density = 128.91 g/L
Therefore, the density of the unknown compound is 128.91 g/L or 0.12891 g/mL (since there are 1000 mL in 1 L).
HQ5.40
Homework Answered Due Today, 11:59 PM
The reaction 3H₂(g) + N₂(g) → 2NH3(g) has an enthalpy of reaction of -92.6 kJ/mol. If 1 g of hydrogen and 2 g of nitrogen are
reacted, how much heat is produced (kJ)?
Answers
The amount of heat energy produced when 1 g of hydrogen and 2 g of nitrogen are reacted, is -6.61 KJ
How do i determine the heat energy produced?First, we shall obtain the limiting reactant. Details below:
3H₂ + N₂ -> 2NH₃
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 g Molar mass of H₂ = 2 g/molMass of H₂ from the balanced equation = 3 × 2 = 6 gFrom the balanced equation above,
28 g of N₂ reacted with 6 g of H₂
Therefore,
2 g of N₂ will react with = (2 × 6) / 28 = 0.43 g of H₂
We can see that only 0.43 g of H₂ is needed in the reaction.
Thus, the limiting reactant is N₂
Finally, we the amount of heat energy produced. Details below:
3H₂ + N₂ -> 2NH₃ ΔH = -92.6 KJ
Molar mass of N₂ = 28 g/molMass of N₂ from the balanced equation = 1 × 28 = 28 gFrom the balanced equation above,
When 28 grams of N₂ reacted, -92.6 KJ of heat energy were produced.
Therefore,
When 2 grams of N₂ will react to produce = (2 × -92.6) / 28 = -6.61 KJ
Thus the heat energy produced from the reaction is -6.61 KJ
Learn more about heat energy:
https://brainly.com/question/31429264
#SPJ1
Which of the following animals are an example of coevolution? *
Answers
Acacia ant and acacias animals are an example of coevolution
Coevolution is the reciprocal evolutionary change in a set of interacting population over time resulting from the interaction between those population and an example of coevolution that is not characteristics of an arm race but one which provides a mutual benefit to both a plant species and insect is that of the acacia ant and acacia plant and many cases of coevolution can be found between plants and insects
Know more about animal
https://brainly.com/question/28711835
#SPJ1
How many atoms or molecules are in 10 grams of table salt?
Answers
Answer:
1.03 x 10²³ atoms NaCl
Explanation:
To find the amount of table salt (NaCl) in atoms, you need to (1) convert grams to moles (using the molar mass) and then (2) convert moles to atoms (using Avogadro's Number). It is important to arrange the ratios/conversions in a way that allows for the cancellation of units.
(Step 1)
Molar Mass (NaCl): 22.99 g/mol + 35.45 g/mol
Molar Mass (NaCl): 58.44 g/mol
10 grams NaCl 1 mole
------------------------ x ----------------------- = 0.17 moles NaCl
58.44 grams
(Step 2)
Avogadro's Number:
6.022 x 10²³ atoms = 1 mole
0.17 moles NaCl 6.022 x 10²³ atoms
-------------------------- x -------------------------------- = 1.03 x 10²³ atoms NaCl
1 mole
What is the orbital or electronic geometry of a molecule with 0 nonbonding electron pairs and 2 bonding electron pairs?
Answers
What atom are molecules but not a compound
Answers
Answer:
Afom of noble gases can exists independently and form monoatomic molecules like helium neon argon etc so these are monoatomic molecules not compound
Explanation:
Given the following data. (i) Ca(s) + 2C(grafite) -> Cacis) X Ca(s) + ⅐0›(g) -> Cao(s) (iit) CaO(s) + H›O(I) -> Ca(OH)(ag) (iv) CHi(g) + 5/20,(8) -> 2C0,(g) + H,0(1) X* (v) C(grafite) + 02(g) -> CO›(g) [4 marks] AH = -62.8 kJ AH = -635.5 kJ AH = -653.1 kJ AH= -1300.0 kJ AH = -393.5 kJ / Calculate AH for the following reaction by using Hess's law and manipulating the given reactions: CaC(s) + H,O(I) - Ca(OH),(ag) + GHa(g) AH = ?
Answers
The enthalpy change (ΔH) for the reaction CaC(s) + H2O(I) → Ca(OH)(ag) + CH4(g) is -3617.6 kJ.
To calculate ΔH for the reaction CaC(s) + H2O(l) → Ca(OH)2(ag) + CH4(g), we can use Hess's law, which states that the enthalpy change of a reaction is independent of the pathway taken and depends only on the initial and final states.
We can manipulate the given reactions to obtain the desired reaction:
(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X (unknown value)
(ii) Ca(s) + 1/2O2(g) → CaO(s) ΔH = -635.5 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
Now, let's manipulate these equations to cancel out the common reactants and products and obtain the desired reaction:
(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X
(ii) Ca(s) + 1/2O2(g) → CaO(s) ΔH = -635.5 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
Now, let's sum up the equations to obtain the desired reaction:(i) Ca(s) + 2C(graphite) → CaC2(s) ΔH = X
(ii) 2Ca(s) + O2(g) → 2CaO(s) ΔH = -1271 kJ
(iii) CaO(s) + H2O(l) → Ca(OH)2(ag) ΔH = -653.1 kJ
(iv) CH4(g) + 2O2(g) → CO2(g) + 2H2O(l) ΔH = -1300.0 kJ
(v) C(graphite) + 1/2O2(g) → CO(g) ΔH = -393.5 kJ
By adding equations (ii), (iii), (iv), and (v), we can cancel out CaO(s), H2O(l), and O2(g):
2Ca(s) + 2C(graphite) + CH4(g) → 2Ca(OH)2(ag) + CO(g) ΔH = X -1271 -653.1 -1300.0 -393.5
2Ca(s) + 2C(graphite) + CH4(g) → 2Ca(OH)2(ag) + CO(g) ΔH = X -3617.6 kJ
For more such questions on enthalpy visit:
https://brainly.com/question/14047927
#SPJ8
What is the mass number of sodium?
Answers
Answer: Since all sodium atoms have 11 protons, this one has 11 protons. This tells us that it also has 11 electrons. Since the mass number is 23, we know that the sum of protons and neutrons in the nucleus must equal 23.
So the answer is : Mass number = 23
Explanation:
Lakesha was culturing bacteria. She used aseptic technique to make sure there was no contamination. When she was
finished, she used water to clean the surface of her table. She then put the sample in the incubator, removed her gloves,
and washed her hands with soap and water.
Which error did Lakesha make in her process?
Answers
Answer: d. She should be using a disinfectant on her table, not just water.
Explanation:
When Lakesha finished culturing the bacteria, she should not have used water alone to clean the surface of the table as water alone would not remove bacteria from the surface of the table if some got there.
She should instead have used a disinfectant on her table as this would have had better chances of significantly reducing the probability of contamination.
Answer:
d on edg
Explanation:
A mixture initially contains A , B , and C in the following concentrations: [A]=0.650mol L−1 , [B]=0.650mol L−1 , and [C]=0.550mol L−1 . The following reaction occurs and equilibrium is established:
A(aq)+2B(aq)⇌C(aq)
At equilibrium, [A]=0.470mol L−1 and [C]=0.730mol L−1 . Calculate the value of the equilibrium constant, Kc .
Express your answer numerically.
Answers
The equilibrium constant of the solution is obtained as 7.
What is the equilibrium constant?We know that the equilibrium constant is the constant that shows how much of the reactant has been converted into products in the reaction. We know that we can be able to use the ICE table to obtain the concentration of the substances at equilibrium.
We know that the concentrations at equilibrium would be;
[A]= 0.470mol L−1
[B]= 0.470mol L−1
[C]=0.730mol L−1
Kc = [C]/[A] [B]^2
Kc = 0.730/(0.470)^3
Kc = 7
Learn more about equilibrium constant:https://brainly.com/question/10038290
#SPJ1
What is the perfect composition of calcium in calcium chloride?
Answers
Answer: 63.96%.
Explanation:
In 111g of Calcium chloride, there is 40g of Calcium and 71g of Chlorine. Percentage Composition of Chlorine is 63.96%.
Hope This Helps!
Igneous rocks would most likely be found near:
Answers
Answer:
the deep sea floor. Known as the oceanic crust.
Explanation:
The deep seafloor (the oceanic crust) is made almost entirely of basaltic rocks, with peridotite underneath in the mantle. Basalts are also erupted above the Earth's great subduction zones, either in volcanic island arcs or along the edges of continents.
Hope this helps :)
Which two mantle hot spots are located at mid-ocean
ridges?
Answers
Answer:
This region of thick, hot crust and mantle extends 1,300 kilometers north and south of the Iceland hotspot and some 1,000 kilometers north and south of the Azores hotspot. Together, the two hotspots appear to be feeding a huge supply of magma to nearly the entire northern segment of the Mid-Atlantic Ridge.
Explanation:
please give me a heart
Answer:
Easter island
A liquid solvent is added to a flask containing an insoluble solid. The total volume of the solid and liquid together is 90.0 mL. The liquid solvent has a mass of 47.3 g and a density of 0.865 g/mL. Determine the mass of the solid given its density is 3.25 g/mL.
Answers
The mass of the solid will be 114.7 g.
Density problemThe density of a substance is given as the ratio of its mass and its volume.
Also, a solid will always displace its own volume in a liquid.
Mass of liquid solvent = 47.3 g
Density of liquid solvent = 0.865 g/mL
Recall that: density = mass/volume
Volume of liquid solvent = mass/density
= 47.3/0.865
= 54.7 mL
Volume of solid + liquid solvent = 90.0 mL
Volume of solid = 90.0 - volume of liquid solvent
= 90.0 - 54.7
= 35.3 mL
Density of solid = 3.25 g/mL
Mass of solid = density x volume
= 3.25 x 35.3
= 114.7 g
The mass of the solid is 114.7 g.
More on density can be found here: https://brainly.com/question/15164682
#SPJ1
Attempt 2
Four marbles are made of different metals. Each marble has the same mass, but a different volume. The density of each
metal is given in the table.
Metal
Density (g/mL)
aluminum
2.70
silver
10.5
rhenium
20.8
nickel
8.90
Place the marbles in order from largest to smallest.
Largest
Answers
Aluminum
V=3/2.70=1.11
Silver
V=3/10.5=.286
Rhenium
V=3/20.8=.144
Nickel
V=3/8.90=.337
This gives us the following list from largest to smallest Aluminum, Nickel, Silver, and Rhenium
The order of marbles can be Aluminum, Nickel, Silver, and Rhenium.
What is volume?If volume is the amount of three-dimensional space contained by a closed surface, such as the amount of space within a given cube, cylinder, or other three-dimensional shape.
Liquid volume is a way to measure an amount of liquid by describing how much three-dimensional space it occupies.
The mass of something is the amount of stuff it is made of. The volume of an object is the amount of space it usually takes up.
Density provides an easy way to calculate a body's mass from its volume or vice versa.
The mass is equal to the volume multiplied by the density (M = Vd), and the volume is equal to the mass divided by the density (V = M/d).
As per the density given, the volume of aluminum can be 1.11mL, Silver is 0.286mL, Rhenium is 0.144mL, Nickel is 0.337mL.
Thus, the order from largest to smallest will be Aluminum, Nickel, Silver, and Rhenium.
For more details regarding volume, visit:
https://brainly.com/question/1578538
#SPJ2
24 of 30
19
20
21
22
23
24
25
26
27
28
Which feature can distinguish between an elemental molecule and a compound molecule?
whether the molecule is formed from at least two atoms
O whether the molecule is formed by the sharing of electrons
O whether the molecule is a gas or a liquid
O whether the molecule is made from different types of atoms
Answers
Answer:
whether the molecule is formed from at least two atoms
Explanation:
A feature that distinguishes an elemental molecule and a compound molecule is whether the molecules are formed from at least two atoms.
An elemental molecule is a molecule that contains two or more of the same kind of elements.
A compound molecule contains two or more different kinds of molecules.
An example of elemental molecule is oxygen gas, O₂. An example of a compound molecule is water H₂O.A molecule is a unit of a compound that can represent such a compound.
Therefore, what distinguishes an elemental molecule from a compound molecule is whether a molecule forms from at least two atoms.
how to synthesize tripropylamine from propylene
Answers
The reactions that result in the emission of light involve the ruthenium label and tripropylamine (TPA), two electrochemically active molecules.
Thus, The electrode surface inside the measurement cell is where the reactions take place.
The ruthenium label is oxidized at the electrode surface as an electrical potential is applied, and TPA is oxidized into a radical cation that spontaneously loses a proton.
When the resultant TPA radical interacts with oxidized ruthenium, the ruthenium label enters an excited state and emits a photon (620 nm) before decaying. The ruthenium label is renewed and ready to carry out numerous light-generating cycles as it goes back to its ground state.
Thus, The reactions that result in the emission of light involve the ruthenium label and tripropylamine (TPA), two electrochemically active molecules.
Learn more about tripropylamine, refer to the link:
https://brainly.com/question/32070784?
#SPJ1
A solution of ammonia and water contains 3.60x1025 water molecules and 6.30x1024 ammonia molecules. How many total hydrogen atoms are in this solution?
Answers
By definition, there are
6.022
×
10
23
such molecules, or
N
A
such molecules in ONE mole of water. And thus in such a quantity there are
N
A
oxygen atoms, and
2
×
N
A
hydrogen atoms...and the mass associated with this numerical quantity of water molecules is approx.
18
⋅
g
...
And so we simply take the quotient....
Moles of water
=
2.60
×
10
23
⋅
water molecules
6.022
×
10
23
⋅
water molecules
⋅
m
o
l
−
1
=
0.432
⋅
m
o
l
...and thus a mass of
0.432
⋅
m
o
l
×
18.01
⋅
g
⋅
m
o
l
−
1
=
7.78
⋅
g
...
Instead of the mole, we could use the dozen, or the gross, or some other numerical quantity ... it just happens that one mole of hydrogen ATOMS have a mass of
1
⋅
g
more or less precisely
HOPE IT HELPS
______ + _______ --> H2O + FrF Complete and balance the equation representing neutralization reaction.
Answers
The general form of a neutralization reaction is HF + FrOH → FrF + H₂O
Which of the following is the formula for a neutralisation reaction?We refer to this as a neutralisation reaction. Only this reaction, which produces NaCl and water as products, is a neutralisation reaction since it involves HCl and NaOH. The resulting response is listed below: NaCl(aq) + H₂O = HCl(aq) + NaOH(aq) (l)
Which of these reactions neutralises an effect?The interaction of H⁺ ions and OH⁻ ions produces water in a neutralisation reaction, which occurs when an acid and a base combine to make water and a salt. The neutralisation of a strong acid and strong base yields a pH of 7.
To know more about reaction visit:-
https://brainly.com/question/28984750
#SPJ1
What tools did you use to collect your data? 1.20 Lab: Earth's Surface Processes.
PLS ILL GIVE U 50 POINTS!!!
Answers
Answer:
I don't know because I don't know about chemistry
Which of the following correctly
states the difference between
elements and compounds?
Elements and compounds are the
same because they are both made of
atoms,
An element is made of two or more
types of atoms combined, while a
compound is made of one type of
stom,
An element is made of one type of
tcom, while a compound is two or
more types of stoms combined.
Answers
Answer:
An element is made of one type of atom, while a compound is two or
more types of atoms combined.
Explanation:
An element is a distinct substance that cannot be split up into simpler substances. Elements are substances that consists of only one kind of atom.
Compounds are substances composed of two or more kinds of atoms/elements joined together in a definite grouping.
There are over a hundred elements known to date. Each one of the elements is symbolized by a capital or a capital letter followed by a small letter derived from English, Latin or Greek Examples are copper, gold, ironThe properties of a compound are distinct and different from those of the individual elements that are combined in its make up. There are several millions of compounds known. They are usually represented by chemical formulae.As we can see, compounds are indeed made up of more than one types of atoms whereas an element is made up of just one single atom.
28
Which substance is made up of many monomers joined together in long chains?
A protein
B
salt
с
ethanol
D
propane
Answers
Answer
B salt this is beacause salt is made of monomers
Which of the following correctly describes a way in which Earth's atmosphere interacts with the biosphere
Answers
Answer:
Living organisms breathe gases in the atmosphere.
Explanation:
Answer:
Living organisms breathe gases in the atmosphere.
Explanation:
Biosphere
the regions of the surface, atmosphere, and hydrosphere of the earth (or analogous parts of other planets) occupied by living organisms.
Atmosphere
the envelope of gases surrounding the earth or another planet
10. A 38.0-g sample of NaOH is dissolved in water, and the solution is diluted to give a final
volume of 1.70 L. The molarity of the final solution is
a. 22.3 M.
b, 0.558 M
c 0.95 M
d. 0.0447 M
e. 0.619 M
Answers
Answer:
B.0.558M
Explanation:
M=n/L
n=m/Mm
Mm=NaOH
=23+16+1
=40g/mol
n=m/Mm
= 38/40
=0.95
M=n/L
=0.95/1.70
=0.558
2. Explain in your own words what will happen to the chemical structure of aspirin when it is in solution at pH 1.0. Compare this to what will happen to the chemical structure of aspirin at pH 8.0. Highlight your answers by drawing appropriate chemical structures for aspirin at each of the pH values.
Answers
Answer:
don't sent such messages whatever you are
According to the concept of solubility and dissociation, aspirin at pH 8 and 10 undergoes dissociation to yield ions.
What are ions?
An ion is defined as an atom or a molecule which has a net electrical charge. There are 2 types of ions :1) cation 2) anion . The cation is the positively charged ion and anion is the negatively charged ion . As they are oppositely charged they attract each resulting in the formation of ionic bond.
Ions consisting of single atom are mono-atomic ions while which consists of two or more ions are called as poly-atomic ions . They are created by chemical interactions . They are very reactive in their gaseous state and rapidly react with oppositely charged ions resulting in neutral molecules.Ions are generated on dissociation.
Learn more about ions,here:
https://brainly.com/question/29183072
#SPJ2
What happens in a reaction if it is at chemical equilibrium?
Responses
The reaction rates of making products and using reactants are equal.
All of the reactants are used up.
The amount of the product is constantly decreasing.
There are no products in the system.
Answers
The reaction can be said to be at equilibrium when the reaction rates of making products and using reactants are equal.
When is a reaction at equilibrium?When the rates of the forward and reverse reactions are equal and the concentrations of the reactants and products don't change over time, a chemical reaction is said to be in equilibrium.
When the system reaches equilibrium, it is in a state of balance, which means that the concentrations of the reactants and products have not changed significantly.
Learn more about reaction equilibrium:https://brainly.com/question/9024475
#SPJ1
What is the scientific word used to describe the process of the protein in the eggs changing form?
Answers
Answer:
Denaturation
Explanation:
How many grams of C5Hg would you need to measure out to have 0.172 mol?
Answers
We would need to measure out approximately 44.84 grams of C5Hg to have 0.172 mol.
To determine the grams of C5Hg needed to have 0.172 mol, we need to use the molar mass of C5Hg and the given amount in moles. The molar mass of a compound represents the mass of one mole of that compound.
The molar mass of C5Hg can be calculated by summing the atomic masses of all the atoms in the compound:
C5Hg: (5 * atomic mass of C) + (1 * atomic mass of Hg)
Using the atomic masses from the periodic table:
Atomic mass of C = 12.01 g/mol
Atomic mass of Hg = 200.59 g/mol
Molar mass of C5Hg = (5 * 12.01 g/mol) + (1 * 200.59 g/mol) = 60.05 g/mol + 200.59 g/mol = 260.64 g/mol
Now, we can calculate the grams of C5Hg needed for 0.172 mol using the mole-to-mass conversion:
Grams of C5Hg = Moles of C5Hg * Molar mass of C5Hg
Grams of C5Hg = 0.172 mol * 260.64 g/mol = 44.84 grams (rounded to two decimal places)
For more such question on mol. visit :
https://brainly.com/question/24191825
#SPJ8
How many moles of O2 are needed to react with 7.00 moles of Al ?
Answers
As per the balanced reaction of Al and oxygen gas, 4 moles of Al needs 3 moles of oxygen gas. Hence, 7 moles of Al needs 5.2 moles of O₂.
What is aluminum oxide ?Aluminum metal is more reactive towards oxygen and it forms oxides on its surface. The balanced chemical equation of the reaction between Al and oxygen is written as follows:
\(\rm 4 Al + 3O_{2}\rightarrow2 Al_{2}O_{3}\)
As per this reaction, 4 moles of aluminum needs 3 moles of oxygen molecule. Then, the number of moles oxygen molecule required to react with 7 moles of Al is :
no.of moles of O₂ = (7×3)/4 = 5.2
Therefore, 5.2 moles of oxygen gas is required to completely react with 7 moles of aluminum metal.
Find more on aluminum oxide:
https://brainly.com/question/9496279
#SPJ1